Teratology and Drugs in Pregnancy
Authors
INTRODUCTION
Teratogenesis refers to the production of defects in the fetus. A teratogenic agent is responsible for producing such a defect. The term teratogen usually is cited in the context of causing anatomical defects in an embryo that was previously differentiating normally.
Teratogens include irradiation, chemicals (drugs), and infectious agents. This chapter reviews principles of teratology and discusses drug use in pregnancy.
DRUGS AND BIRTH DEFECTS
The clinical consequences of drug teratogens must be placed in the overall context of developmental defects in humans. Defects may be from genetic, environmental, or unknown causes. Approximately 25% are known to be genetic in origin (e.g., Mendelian, chromosomal). Approximately 65% of defects are of ostensibly unknown etiology but probably reflect combinations of genetic and environmental factors (polygenic/multifactorial). The risk for malformation after exposure to a drug must be compared with the background rate, which for major malformations in the general population usually is cited as 2–3%. A major malformation is defined as one that is incompatible with survival, such as anencephaly; or one requiring major surgery for correction, such as cleft palate or congenital heart disease; or one producing major dysfunction (e.g., mental retardation). If minor malformations also are included, for example ear tags or extra digits, the rate may be as high as 7–10%. Drug exposure accounts for, at most, 2–3% of birth defects.
Potentially almost any drug used by the mother during pregnancy could be deleterious to the fetus, causing an anatomic defect (teratogenic). Almost all lipid-soluble compounds readily cross the placenta. Water-soluble substances pass more easily when of lower molecular weight. The degree to which a drug is bound to plasma protein also influences the amount of drug that is free to cross the placenta. Overall, most drugs cross the placenta to some degree, with the exception of large organic ions such as heparin (both fractionated and unfractionated) and insulin.
VARIABLES AFFECTING TERATOGENESIS
Specificity of Agent
Some agents are more teratogenic than others. Less obvious is the axiom that an agent may be teratogenic in only certain species. For example, thalidomide produces phocomelia in primates but not in rodents. Within a given species, however, a given teratogen may affect many organ systems. Some organ systems are preferentially affected, but the pattern of anomalies also reflects the organ systems differentiating at the time the agent was administered. For example, administering thalidomide between days 35 and 37 causes ear malformations; administering the agent between days 41 and 44 causes amelia or phocomelia.1
Dosage
Although high doses of a proven teratogen usually are more deleterious than low doses, this is not always true. At any given time, an embryo can respond to a teratogen in one of three ways: (1) at a low dose, there is no effect; (2) at an intermediate dose, a pattern of organ-specific malformations can result; and (3) at a high dose, the embryo may be killed, causing the organ-specific teratogenic action to go unrecognized. In animals, teratogens exert their action within a relatively narrow dose range, usually one-fourth to one-half the average dose that would kill the mother.2 The effect also depends on the developmental stage during which the drug is administered. That is, an agent may be teratogenic only at a higher or lower dose at a different stage. Similarly, at one dose level an agent might be lethal yet not teratogenic, whereas at another level it could be either lethal or teratogenic.
Other variables influence dosage effects. The route of administration must be considered because some agents are teratogenic only if administered in a particular fashion. Such an effect probably is related to absorption phenomena. In addition, small doses administered over several days may produce a different effect than an equal total amount administered at one time. Sequential administration of small doses may induce an enzyme system that can degrade the teratogen, causing less damage than if the entire dose were administered at one time. Conversely, a drug administered sequentially might destroy those cells that catabolize the drug, leading to more deleterious consequences than otherwise might be expected.
Stage of Embryonic Development
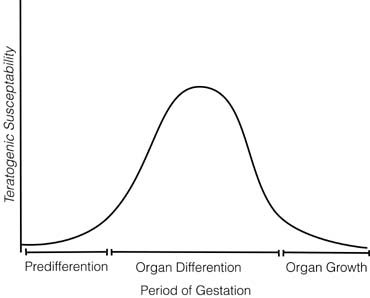
The time during embryogenesis when the fetus is exposed to a potential teratogen is crucial. Three stages of susceptibility may be identified, with these times varying from one organ system to another (Fig. 1): (1) the embryo is relatively resistant to teratogenic insults during the first few weeks of life, perhaps 2 weeks after conception in humans.2 A large insult might kill the embryo, but surviving embryos usually manifest no organ-specific anomalies. Presumably, the explanation is that early embryonic cells have not differentiated irrevocably. If one cell is destroyed, a surviving cell may be able to assume its function; (2) organogenesis, the process of organ differentiation, occurs in most human organ systems between embryonic weeks 3 to 8 (menstrual weeks 5–10); however, differentiating occurs later in the brain and gonads. During organogenesis, susceptibility to teratogens is maximal. Teratogens act in an organ-specific fashion; a teratogen may affect one organ system at one stage of development but another system at another stage. The precise time at which the insult occurs thus determines not only whether a malformation will occur but also the specific spectrum of anomalies. For example, in the rat, 100 rad of radiation produce no anomalies on days 8 or 11 but cause numerous anomalies on day 9 (eye, brain, spinal cord heart, aortic arch, and urinary system) and day 10 (eye, brain, and urinary system) 3; (3) after organogenesis, embryonic development is characterized primarily by increasing organ size. For most human organ systems, this period begins by 8 to 10 embryonic weeks. During this interval, a teratogen can affect the overall growth of the embryo or the size of a specific organ. However, visible malformations are not expected. For example, after the 12th week, administration of androgens to a pregnant woman may produce clitoral enlargement of her female fetus but neither displacement of the urethral orifice or fusion of the labioscrotal folds. In general, a drug that adversely affects a neonate affects an older fetus in a similar fashion. In addition, anomalies can result from secondary effects. For example, cocaine use could lead to vasoconstriction, causing secondary atrophy of a distal organ.
Development of the brain and gonadal tissues continues in the second and third trimesters of pregnancy. Therefore, drug use at this time in pregnancy is a concern, although the effects may not be recognized until later in life. Some of the uterine anomalies resulting from diethylstilbestrol occurred with exposure as late as 20 weeks but were not recognized until after puberty. The brain continues to develop throughout pregnancy and the neonatal period. Fetal alcohol syndrome may occur with chronic exposure to alcohol in the later stages of pregnancy.
Genotype
The genotype of the mother and the fetus influences the efficacy of a teratogen. For example, genotype determines the prevalence of cleft palate in inbred strains of mice whose mothers are administered cortisol during pregnancy.4 Daily administration of 10-mg cortisol during days 11 through 14 produced cleft palate in 100% of offspring of A/Jax parents, in 68% of offspring of C3H parents, and in only 12% of offspring of CBA parents.4 Differences in frequencies of anomalies between various strains presumably are genetic. In humans, only 18% of girls had clitoral hypertrophy after administration of norethindrone to their mothers during a specific time and at a specific dose.5 Another example in humans involves a woman who received diphenylhydantoin during a pregnancy in which she carried dizygotic twins sired by different men.6 The infant sired by a white man showed the hydantoin embryopathy; the infant sired by the black man was normal. Because the environment was identical for the co-twins, any differences in teratogenic effects must reflect genetic differences in susceptibility.
Differences in genetic susceptibility could logically result from either of two well-established genetic mechanisms: polygenic inheritance and monogenic or Mendelian inheritance. Differences between persons in handling drugs, and thus by implication differences in teratogenic susceptibility, probably are usually polygenic. In polygenic inheritance, several genes are assumed to cumulatively affect a given trait. The genotypes produce continuous variability with respect to genetic liability. This mechanism seems especially logical when the steps involved in drug catabolism are recalled: (1) maternal ability to absorb or metabolize a teratogen; (2) placental transfer; and (3) fetal metabolism. Adult monozygotic twins handle drugs more similarly than do dizygotic twins7 but not so similarly as to be explained on the basis of only one gene. However, monogenic factors surely exist. A few persons may be unusually susceptible or unusually resistant to certain drugs because of a single mutant allele. Such persons are said to have a pharmacogenetic disorder. Examples include pseudocholinesterase deficiency, warfarin resistance, heparin resistance, and inability to catabolize (decarboxylate) drugs such as hydralazine or isoniazid. By analogy, a mutant allele could render a fetus incapable of inactivating a potential teratogen. Administration of a specific teratogen thus might adversely affect that fetus but not other (normal) fetuses. The clinical significance is that certain individuals (fetuses) may be unusually sensitive to certain teratogens.
The ability to identify individuals at increased risk for untoward effects is a major goal of the pharmaceutical industry. Current strategies are focusing on finding single nucleotide polymorphisms (SNP) conferring such characteristics.
Drug Interactions
Simultaneous administration of two teratogens may produce a different effect from that existing when the two are administered separately. For example, folic acid reduces the frequency of cortisol-induced teratogenesis in mice,8 possibly because of induction of enzyme systems that catabolize the teratogen or compete for binding sites. Conversely, one agent may enhance the teratogenic potential of another. For example, the food preservative benzoic acid enhances aspirin teratogenicity in rats.9 Possible mechanisms include enzyme inhibition, destruction of enzyme-producing cells, and saturation of binding sites on carrier proteins that, if available, would decrease levels of the unbound active teratogen.
Other Factors
Variability in teratogenic response sometimes is associated with other environmental or morphologic factors: maternal or fetal weight, in utero position of the fetus, proximity to other affected litter mates, uterine vasculature, and diet. However, further investigation usually reveals that these factors are correlated with other factors already cited. For example, the inverse correlation between maternal weight and susceptibility of the fetus to cortisol-induced cleft palate is related not to weight per se but to dose per unit mass.10
CELLULAR ACTION OF A TERATOGEN
A teratogen may potentially affect embryogenesis by causing gene mutation, chromosome breakage or nondisjunction, depletion or inhibition of precursors or substrates, depletion of energy sources, inhibition of enzymes, or changes in intracellular milieu secondary to changes in membrane integrity.2 These lead to cell death, reduced cell division, failure of expected interaction between cells, disruption of cell migration, or mechanical disruption.
Regardless of the initial mechanism or the intermediary effect, the ultimate result usually is an organ with too few cells. The critical mass necessary for induction or continuation of differentiation is lacking; thus, the particular organ system fails to develop.11 Of course, a few anomalies (e.g., polydactyly or labioscrotal fusion) could result either from increased cell proliferation or from failure of localized cell degeneration.
PROOF OF TERATOGENICITY
Teratogens usually are first identified by alert clinicians. Unfortunately, many agents are falsely implicated, requiring case reports to be assessed critically. Retrospective case-control designs thus are commonly used. Such an experimental design is efficient in identifying teratogens but vulnerable to false-positive conclusions, because recall biases and memory biases render control and subject (mothers of affected infants) unequal in incentive. That is, normal controls have less incentive to recollect events than women having anomalous infants (an “anomaly control,” a woman having an abnormal outcome, but not that being tested, can be used to minimize this problem). However, definitive cohort (prospective) studies are expensive and complex. Thus, there is no ideal way of assessing teratogens. A variety of approaches can be used, usually leading to a scientific consensus eventually. Confounding any study is knowledge that similar congenital anomalies occur in women not exposed to teratogens.
Given these caveats, it is not surprising that proof of teratogenicity is difficult. Observations such as the following can implicate a particular agent: (1) the agent was associated more often with subjects having a particular anomaly than with suitable controls; (2) an anomaly or pattern of anomalies is consistently associated with the suspected teratogen; (3) the agent was presented during the stage of organogenesis when the anomaly would have been likely to occur; (4) the anomaly was less common before the time the potential teratogen was available (e.g., phocomelia was almost unreported before the time thalidomide was introduced); and (5) the anomaly can be produced in experimental animals by administration of the agent during a stage of organogenesis comparable with that believed to be involved in causing the anomaly in humans. (However, negative animal data do not prove that a drug is innocuous to humans.)
Epidemiological pitfalls in assessing human teratogens are myriad, and several different surveillance methods are used. No single method or design is universally reliable.12,13,14
LABELING CATEGORIES
The United States Food and Drug Administration (FDA) lists five categories of labeling for drug use in pregnancy: (A) controlled studies in women fail to demonstrate a risk to the fetus in the first trimester, and the possibility of fetal harm appears remote; (B) animal studies do not indicate a risk to the fetus; there are no controlled human studies or animal studies do show an adverse effect on the fetus, but well-controlled studies in pregnant women have failed to demonstrate a risk to the fetus; (C) studies show the drug to have animal teratogenic or embryocidal effects, but no controlled studies are available in women, or no studies are available in either animals or women; (D) positive evidence of human fetal risk exists, but benefits in certain situations (e.g., life-threatening situations or serious diseases for which safer drugs cannot be used or are ineffective) may make use of the drug acceptable despite its risks; and (E) studies in animals or humans have demonstrated fetal abnormalities, or evidence demonstrates fetal risk based on human experience, or both, and the risk clearly outweighs any possible benefit.
The Teratology Society suggests abandoning the FDA classification.15 The categories imply that risk increases from category A to X, whereas the drugs in different categories may pose similar risks but be in different categories based on risk–benefit considerations. Second, the categories create the impression that drugs within a category present similar risks, whereas the category definition permits inclusion in the same category drugs that vary in type, degree or extent of risk. All new medications are classified as category C, leading to an elevated impression of the danger of the medication. When counseling patients or responding to queries from physicians, we recommend avoiding the PDR and instead using specific descriptions in teratogen databases to provide accurate information.
An FDA concept paper was presented on May 20, 1999, which outlined a model for labeling and included suggested sections titled, “Clinical management statement,” “Summary risk assessment,” and “Discussion of data” for both pregnancy and lactation sections,16 but this has not yet been implemented.
Patients should be educated about avenues other than the use of drugs to cope with tension, aches and pains, and viral illnesses during pregnancy. Drugs should be used only when necessary. The risk-to-benefit ratio should justify the use of a particular drug, and the minimum effective dose should be used. Because long-term effects of drug exposure may not be revealed for many years, caution with regard to the use of any drug in pregnancy is warranted
Teratogen Information Services
An organization of teratology information services and several computer databases are available to physicians who counsel pregnant women (Table 1).
Table 1. Teratogen information services
| Organization of Teratogen Information Services: |
| http://orpheus.ucsd.edu/ctis; telephone: 801–328–2229 |
| Computer databases |
| MICROMEDEX, Inc., 6200 South Syracuse Way, Suite 300, Englewood, CO 80111–4740 |
| Reproductive Toxicology Center, REPROTOX, Columbia Hospital for Women Medical Center, 2440 M Street NW, Suite 217, Washington, DC 20037–1404 |
| Teratogen Information Service, TERIS, University of Washington, Office of Technology Transfer, 4225 Roosevelt Way NE, Suite 301, Seattle, WA 98105 |
EFFECTS OF THERAPEUTIC DRUGS
Vitamin A Congeners
Isotretinoin (Accutane) is a significant human teratogen. This drug is marketed for treatment of cystic acne and unfortunately has been used inadvertently by women who were not planning pregnancy.17 It is labeled as contraindicated in pregnancy (FDA category X) with appropriate warnings that a negative pregnancy test result is required before therapy. The risk for structural anomalies in patients studied prospectively is estimated to be approximately 25%. An additional 25% have mental retardation alone.18 The infants with malformations have a characteristic pattern of craniofacial, cardiac, thymic, and central nervous system anomalies. They include microtia or anotia (small or absent ears), micrognathia, cleft palate, heart defects, thymic defects, retinal or optic nerve anomalies, and central nervous system malformations, including hydrocephalus.17 Microtia is rare as an isolated anomaly yet appears commonly as part of the retinoic acid embryopathy. Cardiovascular defects include great vessel transposition and ventricular septal defects.
Unlike vitamin A, isotretinoin is not stored in tissue. Therefore, a pregnancy after discontinuation of isotretinoin is not at risk, because the drug is no longer detectable in serum 5 days after its ingestion. In 88 pregnancies prospectively ascertained after discontinuation of isotretinoin, no increased risk of anomalies was noted in contrast to etretinate.19
Topical tretinoin (Retin-A) has not been associated with any teratogenic risk.20
Etretinate (Tegison) is marketed for use in psoriasis and may have a teratogenic risk similar to that of isotretinoin. Case reports of malformation, especially central nervous system,21 have appeared, but the absolute risk is unknown. The half-life of several months makes levels cumulative, and cases have occurred in which the drug still was detectable as late as 2.9 years after the last dose. The drug is contraindicated in women of reproductive age.
There is no evidence that vitamin A itself in normal doses is teratogenic, nor is beta-carotene. The levels in prenatal vitamins (5000 IU/day orally) have not been associated with any documented risk. Eighteen cases of birth defects have been reported after exposure to levels of 25,000 IU of vitamin A or greater during pregnancy. Vitamin A in doses greater than 10,000 IU per day was shown to increase the risk of malformations in one study22 but not in another.23
ANTINEOPLASTIC DRUGS AND IMMUNOSUPPRESSANTS
Methotrexate
Methotrexate, a folic acid antagonist, appears to be a human teratogen, although experience with it is limited. Infants of three women known to receive methotrexate in the first trimester of pregnancy had multiple congenital anomalies, including cranial defects and malformed extremities. Eight normal infants were delivered to seven women treated with methotrexate in combination with other agents after the first trimester. When low-dose oral methotrexate (7.5 mg/wk) was used for rheumatoid disease in the first trimester, five full-term infants were normal, and three patients experienced spontaneous abortions.24
Azathiprine
Azathioprine (Imuran) has been used by patients with renal transplants or systemic lupus erythematosus. The frequency of anomalies in 80 women treated in the first trimester was not increased.25 Two infants had leukopenia, one was small for gestational age, and the others were normal. A review of more than 50 studies shows no increase in anomalies.26
Cyclosporine
No increased risk of anomalies in fetuses exposed to cyclosporine (Sandimmune) in utero has been reported.27 An increased rate of prematurity and growth restriction also has been noted, but it is difficult to separate the contributions of the underlying disease and the drugs given to these transplant patients. The B-cell line may be depleted more than the T-cell line,28 and one author recommends that infants exposed to immunosuppressive agents be followed-up for possible immunodeficiency.
Cyclophosphamide
Ten infants with malformations have resulted from first-trimester exposure to cyclophosphamide (Cytoxan), but these infants also were exposed to other drugs or radiation.29 Low birth weight may be associated with use after the first trimester, but this also may reflect the underlying medical problem.
Chloroquine
Chloroquine (Aralen) is safe in doses used for malarial prophylaxis, and there was no increased incidence of birth defects among 169 infants exposed to 300 mg once weekly.30 However, after exposure to larger anti-inflammatory doses (250–500 mg/day), two cases of cochleovestibular paresis were reported.31 No abnormalities were noted in an additional 14 infants.32
When cancer chemotherapy is used during embryogenesis, there is an increased rate of spontaneous abortion and major birth defects. Later in pregnancy, there is a greater risk of stillbirth and intrauterine growth restriction, and myelosuppression often is present in the infant.33
ANTICONVULSANTS
Phenytoin, Valproic Acid, Carbamazepine
Women with epilepsy who use anticonvulsants during pregnancy have approximately double the general population risk for malformations. Compared with the general risk of 2–3%, the risk for major malformations in epileptic women using anticonvulsants is approximately 5%, especially cleft lip with or without cleft palate and congenital heart disease. Valproic acid (Depakene) and carbamazepine (Tegretol) each have approximately a 1% risk of neural tube defects and possibly other anomalies.34, 35 In addition, the offspring of epileptic women have a 2–3% incidence of epilepsy, five-times that in the general population.
Holmes and colleagues36 screened 128,049 pregnant women at delivery to identify three groups of infants: those exposed to anticonvulsant drugs, those unexposed to anticonvulsant drugs but with a maternal history of seizures, and those unexposed to anticonvulsant drugs with no maternal history of seizures (control group). The infants were examined systematically for the presence of malformations. The combined frequency of anticonvulsant embryopathy was higher in 223 infants exposed to one anticonvulsant drug than in 508 control infants (20.6 vs. 8.5%; odds ratio: 2.8; 95% confidence interval: 1.1–9.7). The frequency was also higher in 93 infants exposed to two or more anticonvulsant drugs than in the controls (28.0 vs. 8.5%; odds ratio: 4.2; 95% confidence interval: 1.1–5.1). A greater number of anticonvulsants increase the risk of malformation. The 98 infants whose mothers had a history of epilepsy but used no anticonvulsant drugs during the pregnancy did not have a higher frequency of those abnormalities than the control infants. A distinctive pattern of physical abnormalities in infants of mothers with epilepsy is associated with the use of anticonvulsant drugs during pregnancy, rather than with epilepsy itself.
Possible causes of anomalies in epileptic women using anticonvulsants include a genetic predisposition to both epilepsy and malformations, genetic differences in drug metabolism, the specific drugs themselves, and deficiency states induced by drugs such as decreased folate levels. Phenytoin (Dilantin) decreases folate absorption and lowers the serum folate, which has been implicated in birth defects.37 Therefore, folic acid supplementation should be given to these mothers, but this may require adjustment of the anticonvulsant dose. Most authorities recommend 4 mg per day of folic acid for women at high risk.38 One retrospective study suggests that folic acid at doses of 2.5–5 mg daily could reduce birth defects in women using anticonvulsant drugs.39
Fewer than 10% of offspring show the fetal hydantoin syndrome,40 which consists of microcephaly, growth deficiency, developmental delays, mental retardation, and dysmorphic craniofacial features, orofacial, cardiovascular, and digital malformations. The risk may be as low as 1% to 2%.41 Whereas several of these features also are found in other syndromes, such as fetal alcohol syndrome, more common in the fetal hydantoin syndrome are hypoplasia of the nails and distal phalanges, and hypertelorism. Carbamazepine (Tegretol) also is associated with an increased risk of a dysmorphic syndrome.42
A genetic metabolic defect in arene oxide detoxification in the infant may increase the risk for a major birth defect.43 Epoxide hydrolase deficiency may indicate susceptibility to fetal hydantoin syndrome.44
In a follow-up study of long-term effects of antenatal exposure to phenobarbital and carbamazepine, anomalies were not related to specific maternal medication exposure. There were no neurologic or behavioral differences between the two groups.45 However, children exposed in utero to phenytoin scored 10 points lower on intelligence quotient (IQ) tests than children exposed to carbamazepine or nonexposed controls.46 Also, prenatal exposure to phenobarbital decreased verbal IQ scores in adult men.47
NEWER ANTIEPILEPTIC DRUGS
Lamotrigine (Lamictal) has been studied in a registry48 established by the manufacturer, GlaxoWellcome. Eight of 123 infants (6.5%; 95% confidence interval: 3.1–12.8) born to women treated with lamotrigine during the first trimester and followed-up prospectively to birth were found to have congenital anomalies. All of the mothers of children with malformations had a seizure disorder, and some used at least one other medication during the pregnancy.
Lamotrigine is an inhibitor of dihydrofolate reductase and decreases embryonic folate levels in experimental animals, so it is theoretically possible that this drug would be associated with an increased malformation risk, like the other antiepileptic drugs.49 The limited human data do not indicate a major risk for congenital malformations or fetal loss after first-trimester exposure to lamotrigine.50
Some women may have used anticonvulsant drugs for a long period without reevaluation of the need for continuation of the drugs. For patients with idiopathic epilepsy who have been seizure-free for 2 years and who have a normal electroencephalogram, it may be safe to attempt a trial of withdrawal of the drug before pregnancy.51
Most authorities agree that the benefits of anticonvulsant therapy during pregnancy outweigh the risks of discontinuation of the drug if the patient is first examined during pregnancy. The blood level of drug should be monitored to ensure a therapeutic level but minimize the dosage. If the patient has not been using her drug regularly, a low blood level may demonstrate her lack of compliance, and she may not need the drug. Because the albumin concentration decreases during pregnancy, the total amount of phenytoin measured is decreased, because it is highly protein-bound. However, the level of free phenytoin, which is the pharmacologically active portion, is unchanged. Neonatologists need to be notified when a patient is using anticonvulsants because this therapy can affect vitamin K-dependent clotting factors in the newborn. Vitamin K supplementation at 10 mg daily for these mothers has been recommended for the last month of pregnancy.52 Because hypothyroidism in pregnancy may adversely effect neuropsychological development of the fetus,53 it is prudent to monitor thyroid function throughout pregnancy and to adjust the thyroid dose to maintain a normal TSH level. Topical iodine preparations are readily absorbed through the vagina during pregnancy, and transient hypothyroidism has been demonstrated in the newborn after exposure during labor.
ANTICOAGULANTS
Warfarin
Warfarin (Coumadin) has been associated with chondrodysplasia punctata, which is similar to the genetic Conradi-Hunerman syndrome. This syndrome, occurring in approximately 5% of exposed pregnancies, includes nasal hypoplasia, bone stippling seen on radiologic examination, ophthalmologic abnormalities including bilateral optic atrophy, and mental retardation. The ophthalmologic abnormalities and mental retardation may occur,54 even with use only beyond the first trimester. Fetal and maternal hemorrhage also have been reported in pregnant women on warfarin, although the incidence can be decreased with careful control of the prothrombin time.
Heparin
The alternative drug heparin does not cross the placenta, because it is a large molecule with a strong negative charge. Because heparin does not have an adverse effect on the fetus when administered during pregnancy, it should be the drug of choice for patients requiring anticoagulation. In one study, heparin treatment was associated with more thromboembolic complications and more bleeding complications than warfarin therapy.55 Therapy with 20,000 U per day for more than 20 weeks also has been associated with bone demineralization.56 Thirty-six percent of patients had more than a 10% decrease from baseline bone density to postpartum values.57 The risk for spine fractures was 0.7% with low-dose heparin and 3% with a high-dose regimen.58 Heparin also can cause thrombocytopenia.
Low-Molecular-Weight Heparins
Low-molecular-weight heparins may have benefits over standard unfractionated heparin.59 The molecules still are relatively large and do not cross the placenta.60 The half-life is longer, allowing for once-daily administration. However, enoxaparin is cleared more rapidly during pregnancy, so twice-daily dosing may be advised. They have a much more predictable dose–response relationship, obviating the need for monitoring. There is less risk for heparin-induced thrombocytopenia and clinical bleeding at delivery, but studies suggesting less risk for osteoporosis are preliminary. The cost is substantially higher than that for standard heparin.61, 62
The risks of heparin during pregnancy may not be justified in patients with only a single episode of thrombosis in the past.63, 64 Conservative measures should be recommended, such as elastic stockings and avoidance of prolonged sitting or standing.
In patients with cardiac valve prostheses, full anticoagulation usually with coumadin65 is necessary, because low-dose heparin resulted in three valve thromboses (two fatal) in 35 mothers so treated.66
Thyroid and Antithyroid Drugs
Propylthiouracil (PTU) and methimazole (Tapazole) both cross the placenta and may cause some degree of fetal goiter. In contrast, the thyroid hormones triiodothyronine (T3) and thyroxine (T4) cross the placenta poorly, so that fetal hypothyroidism produced by antithyroid drugs cannot be corrected satisfactorily by administration of thyroid hormone to the mother. Thus, the goal of such therapy during pregnancy is to keep the mother slightly hyperthyroid to minimize fetal drug exposure. Methimazole has been associated with scalp defects (aplasia cutis) in infants67 and a higher incidence of maternal side effects. However, PTU and methimazole are equally effective and safe for therapy of hyperthyroidism, and the risk for aplasia cutis is small.68
Radioactive iodine administered for thyroid ablation or for diagnostic studies is not concentrated by the fetal thyroid until after 12 weeks of pregnancy.69 Thus, with inadvertent exposure, usually around the time of missed menses, there is no specific risk to the fetal thyroid from iodine (I)-131 or iodine (I)-125 administration.
The need for thyroxine increases in many women with primary hypothyroidism when they are pregnant, as reflected by an increase in serum thyrotropin (TSH) concentrations. In one study, the mean thyroxine dose before pregnancy was 0.10 mg per day; it was increased to a mean dose of 0.15 mg per day during pregnancy.70, 71
Psychoactive Drugs
There is no clear risk documented for most psychoactive drugs with respect to overt birth defects. However, effects of chronic use of these agents on the developing brain in humans are difficult to study, so a conservative attitude is appropriate. Lack of overt defects does not exclude the possibility of behavioral teratology.
TRANQUILIZERS
Conflicting reports of the possible teratogenicity of the various tranquilizers, including meprobamate (Miltown) and chlordiazepoxide (Librium), have appeared, but in prospective studies no risk for anomalies has been confirmed.72 In most clinical situations, the risk-to-benefit ratio does not justify the use of benzodiazepines in pregnancy.
A fetal benzodiazepine syndrome has been reported in seven infants of 36 mothers who regularly used benzodiazepines during pregnancy.73 However, the high rate of abnormality occurred with concomitant alcohol and substance abuse and may not be caused by the benzodiazepine exposure.74 Perinatal use of diazepam (Valium) has been associated with hypotonia, hypothermia, and respiratory depression.
LITHIUM
In the International Register of Lithium Babies,75 217 infants are listed as exposed to lithium (Eskalith, Lithobid) at least during the first trimester of pregnancy, and 25 (11.5%) had malformations. Eighteen had cardiovascular anomalies, including six cases of the rare Ebstein anomaly, which occurs only once in 20,000 in the nonexposed population. Of 60 unaffected infants who were followed-up until age 5 years, no increased mental or physical abnormalities were noted compared with unexposed siblings.76
However, two other reports suggest that there was a bias of ascertainment in the registry and that the risk of anomalies is lower than previously thought. A case-control study of 59 patients with Ebstein anomaly showed no difference in the rate of lithium exposure in pregnancy from a control group of 168 children with neuroblastoma.77 A prospective study of 148 women exposed to lithium in the first trimester showed no difference in the incidence of major anomalies compared with controls.78 One fetus in the lithium-exposed group had Ebstein anomaly, and one infant in the control group had a ventricular septal defect. The authors conclude that lithium is not a major human teratogen. Nevertheless, we recommend that women exposed to lithium be offered ultrasound and fetal echocardiography.
Lithium is excreted more rapidly during pregnancy; thus, serum lithium levels should be monitored. Perinatal effects of lithium have been noted, including hypotonia, lethargy, and poor feeding in the infant. Also, complications similar to those seen in adults on lithium have been noted in newborns, including goiter and hypothyroidism.
Two cases of polyhydramnios associated with maternal lithium treatment have been reported.79, 80 Because nephrogenic diabetes insipidus has been reported in adults using lithium, the presumed mechanism of this polyhydramnios is fetal diabetes insipidus. Polyhydramnios may be a sign of fetal lithium toxicity.
It is usually recommended that drug therapy be changed in pregnant women using lithium to avoid fetal drug exposure. Tapering over the course of 10 days delays the risk for relapse.81 However, discontinuing lithium is associated with a 70% chance for relapse of the affective disorder in 1 year as opposed to 20% in those who continue to use lithium. Discontinuation of lithium may pose an unacceptable risk of increased morbidity in women who have had multiple episodes of affective instability. These women should be offered appropriate prenatal diagnosis with ultrasound, including fetal echocardiography.
ANTIDEPRESSANTS
Women who discontinue antidepressants because of pregnancy have a relapse rate of depression of 68%,82 and so pregnancy should not be considered protective.
Imipramine (Tofranil) was the original tricyclic antidepressant claimed to be associated with cardiovascular defects, but few patients have been studied. Of 75 newborns exposed in the first trimester, six major defects were observed, three being cardiovascular.83
Amitriptyline (Elavil) has been used more widely. Although occasional reports associate the use of this drug with birth defects, most of the evidence supports its safety. In the Michigan Medicaid study, 467 newborns had been exposed during the first trimester, with no increased risk for birth defects.84
No increased risk for major malformations has been found after first-trimester exposure to fluoxetine (Prozac) in more than 500 pregnancies.85, 86
Nulman and colleagues87 evaluated the neurobehavioral effects of long-term fluoxetine exposure during pregnancy and found no abnormalities among 228 children aged 16 to 86 months (average age: 3 years). Theoretically, some psychiatric or neurobehavioral abnormality might occur as a result of exposure, but it would be difficult to ascertain because of all of the confounding variables.
Chambers and associates88 found more minor malformations and perinatal complications among infants exposed to fluoxetine throughout pregnancy, but this study is difficult to interpret because the authors did not control for depression. When a group whose mothers received tricyclic agents was used as a control for depression, infants exposed to fluoxetine in utero did not have more minor malformations or perinatal complications.85
The current data on SSRI use during pregnancy are conflicting, with some studies showing no increased risk of birth defects.89, 90, 91, 92 Other investigators have shown a small increased risk of cardiac defects with paroxetine,93 and the manufacturer has changed the FDA category from C to D.
The newer selective serotonin receptor inhibitors, fluvoxamine (Luvox), sertraline (Zoloft), and citalopram (Celexa), have also been studied.89 No increased risks for major malformation, language, or behavioral development abnormalities have been found.90, 91
A syndrome of withdrawal in the neonate after in utero exposure to SSRIs has been described.94 The rate of persistent pulmonary hypertension was 1%, a six-fold increase over non-exposed infants.
A detailed summary reviewing use of psychiatric medications during pregnancy and lactation has recently been published by the American College of Obstetricians and Gynecologists.95
ANTIEMETICS
Remedies suggested to help nausea and vomiting in pregnancy without pharmacologic intervention include eating crackers at the bedside on first awakening in the morning before getting out of bed, getting up slowly, omitting the use of iron tablets or multivitamins containing iron, consuming frequent small meals, and eating protein snacks at night. Faced with a self-limited condition occurring at the time of organogenesis, the clinician is well advised to avoid the use of medications when possible and to encourage these supportive measures initially.
VITAMIN B6
Vitamin B6 (pyridoxine), 25 mg or 10 mg three times daily, has been reported in two randomized placebo-controlled trials to be effective for treating the nausea and vomiting of pregnancy.96, 97 In several other controlled trials, there was no evidence of teratogenicity.
ANTIHISTAMINES
Doxylamine (Unisom) is an effective antihistamine for nausea in pregnancy and can be combined with vitamin B6 to produce a therapy similar to the former preparation, Bendectin. Vitamin B6 (25 mg) and doxylamine (Unisom) 25 mg at bedtime and one-half of each in the morning and afternoon, is an effective combination.
In one randomized placebo-controlled study, meclizine (Bonine) gave significantly better results than placebo.98 Prospective clinical studies provide no evidence that meclizine is teratogenic in humans. In 1014 patients in the Collaborative Perinatal Project99 and an additional 613 patients from the Kaiser Health Plan,100 no teratogenic risk was found.
No teratogenicity has been noted with dimenhydrinate (Dramamine), but a 29% failure rate and a significant incidence of side effects, especially drowsiness, has been reported.101 In 595 patients treated in the Collaborative Perinatal Project, no teratogenicity was noted with diphenhydramine (Benadryl).99 Drowsiness can be a problem.
Trimethobenzamide
Trimethobenzamide (Tigan), an anti-nausea medicine not classified as either an antihistamine or a phenothiazine, has been used for nausea and vomiting in pregnancy. The data collected from the few patients are conflicting. In 193 patients in the Kaiser Health Plan study100 exposed to trimethobenzamide, there was a suggestion of increased congenital anomalies (p < 0.05); no concentration of specific anomalies was observed in these children, however, and some of the mothers also used other drugs. In 340 patients in the Collaborative Perinatal Project,99 no evidence for an association between this drug and malformations was found.
Phenothiazines
Because of the potential for severe side effects, the phenothiazines have not been used routinely in the treatment of mild or moderate nausea and vomiting but have been reserved for the treatment of hyperemesis gravidarum. Chlorpromazine (Thorazine) has been shown to be effective in hyperemesis gravidarum, with the most important side effects being drowsiness and the potential for extrapyramidal symptoms.
Teratogenicity does not appear to be a problem with the phenothiazines, including prochlorperazine (Compazine), chlorpromazine (Thorazine) and perphenazine (Trilafon). In the Kaiser Health Plan study,100 976 patients were treated, and in the Collaborative Perinatal Project,99 1309 patients were treated; no evidence of association between these drugs and malformations was noted.
Metoclopramide
Metoclopramide (Reglan) can be effective therapy for nausea and vomiting in pregnancy. The frequency of malformations was no greater than expected among the infants of 190 women who had been given prescriptions for metoclopramide during the first trimester of pregnancy in a Danish record linkage study.102
Ondanestron
Ondansetron (Zofran) is no more effective than promethazine (Phenergan), has not been evaluated for teratogenicity, and is considerably more costly.103
Methylprednisolone
Forty patients with hyperemesis admitted to the hospital were randomized to oral methylprednisolone or oral promethazine, and methylprednisolone was more effective.104 It is recommended that corticosteroids be used only after the first trimester because of a potential increase in cleft lip and/or palate in the infant.105, 106
Ginger
Ginger has also been shown to be an effective treatment for nausea and vomiting during pregnancy in two placebo-controlled trials.107, 108 Ginger capsules, 250 mg four times per day, decreased nausea and vomiting in outpatients and in hospitalized patients with hypermesis gravidarum.
Acid-Suppressing Drugs
The use of cimetidine, omeprazole, and ranitidine has not been found to be associated with any teratogenic risk in 2261 exposures.109
ANTIHISTAMINES AND DECONGESTANTS
No increased risk for anomalies has been associated with most of the commonly used antihistamines, such as chlorpheniramine (Chlor-Trimeton). However, in the Collaborative Perinatal Project,99 the risk for malformations increased after exposure to brompheniramine (Dimetane) (10/65 infants), an effect that was not found for other antihistamines.
Terfenadine (Seldane) has been associated in one study with an increased risk for polydactyly, although another study found no increased risk.110 Astemizole (Hismanal) did not increase the risk for birth defects in 114 infants exposed in the first trimester.111
An association between exposure during the last 2 weeks of pregnancy to antihistamines in general and retrolental fibroplasia in premature infants has been reported.112
In the Collaborative Perinatal Project,99 an increased risk for birth defects was noted with phenylpropanolamine (Entex LA) exposure in the first trimester. In one retrospective study, an increased risk for gastroschisis was associated with first-trimester use of pseudoephedrine (Sudafed).113 Although these findings have not been confirmed, use of these drugs for trivial indications should be discouraged because long-term effects are unknown. If decongestion is necessary, topical nasal sprays result in a lower dose to the fetus than systemic medication.
Patients should be educated that antihistamines and decongestants are only symptomatic therapy for the common cold and have no influence on the course of the disease. Other remedies should be recommended, such as use of a humidifier, rest, and fluids. If medications are necessary, combinations with two drugs should not be used if only one drug is necessary. If the situation is truly an allergy, an antihistamine alone should suffice.
MILD ANALGESICS
Pregnant patients should be encouraged to use nonpharmacologic remedies such as local heat and rest before taking medications.
Aspirin
There is no evidence of any teratogenic effect of aspirin used in the first trimester for most defects.99, 114 However, one study suggested an increased risk for gastroschisis.115 Aspirin does have significant perinatal effects, however, because it inhibits prostaglandin synthesis. Uterine contractility is decreased, and patients using aspirin in analgesic doses have delayed onset of labor, longer duration of labor, and an increased risk for a prolonged pregnancy.116
Aspirin also decreases platelet aggregation, which can increase the risk for bleeding before and at delivery. Platelet dysfunction has been described in newborns within 5 days of ingestion of aspirin by the mother.117 Because aspirin causes permanent inhibition of prostaglandin synthetase in platelets, the only way for adequate clotting to occur is for more platelets to be produced. Multiple organs may be affected by chronic aspirin use. Prostaglandins mediate the neonatal closure of the ductus arteriosus. In one case report, maternal ingestion of aspirin close to the time of delivery was related to closure of the ductus arteriosus in utero.118
Low-dose aspirin ultimately may prove beneficial in preventing fetal wastage associated with autoimmune diseases. Cowchock and associates119 report that in patients with antiphospholipid-associated fetal wastage, heparin is preferable to prednisone in combination with low-dose aspirin, and low-dose aspirin along may be sufficient.120
Acetaminophen
Acetaminophen (Tylenol, Datril) also has shown no evidence of teratogenicity.121 With acetaminophen, inhibition of prostaglandin synthesis is reversible, so that once the drug has cleared, platelet aggregation returns to normal. The bleeding time is not prolonged with acetaminophen in contrast to aspirin,122 and the drug is not toxic to the newborn. Thus, if a mild analgesic or antipyretic is indicated, acetaminophen is preferred. Absorption and disposition of acetaminophen in normal doses is not altered by pregnancy.123
Other Nonsteroidal Anti-Inflammatory Drugs
No evidence of teratogenicity has been reported for other nonsteroidal anti-inflammatory drugs (e.g., ibuprofen124 [Motrin, Advil], naproxen,125 [Naprosyn]), but limited information is available. Use for acute pain for less than 48 hours is recommended. Chronic use may lead to oligohydramnios, and constriction of the fetal ductus arteriosus or neonatal pulmonary hypertension, as has been reported with indomethacin, might occur.
Propoxyphene
Propoxyphene (Darvon) is an acceptable alternative mild analgesic with no known teratogenicity.99 However, it should not be used for trivial indications because it has potential for narcotic addiction. Evidence of risk in late pregnancy comes from case reports of infants of mothers who were addicted to propoxyphene and had typical narcotic withdrawal in the neonatal period.126
Codeine
In the Collaborative Perinatal Project, no increased relative risk of malformations was observed in 563 codeine users.99 Codeine can cause addiction and newborn withdrawal symptoms if used to excess perinatally.
Sumatriptan
Of 183 exposures to sumatriptan (Imitrex) in the first trimester,48 seven infants (3.8%) had birth defects, which was not significantly different from the nonexposed population.127
ANTIBIOTICS AND ANTI-INFECTIVE AGENTS
Because pregnant patients are particularly susceptible to vaginal yeast infections, antibiotics should be used only when clearly indicated. Therapy with antifungal agents may be necessary during or after the course of therapy.
Penicillins
Penicillin, ampicillin, and amoxicillin (Amoxil) are safe during pregnancy. In the Collaborative Perinatal Project,99 3546 mothers used penicillin derivatives in the first trimester of pregnancy, with no increased risk for anomalies. There is little experience in pregnancy with the newer penicillins such as piperacillin (Pipracil) and mezlocillin (Mezlin). These drugs, therefore, should be used in pregnancy only when another better-studied antibiotic is not effective.
Most penicillins are primarily excreted unchanged in the urine, with only small amounts being inactivated in the liver. Patients with impaired renal function require a reduction in dosage.
Serum levels of the penicillins are lower and their renal clearance is higher throughout pregnancy than in the nonpregnant state. The increase in renal blood flow and glomerular filtration rate results in increased excretion of these drugs. The expansion of the maternal intravascular volume during the late stages of pregnancy is another factor that affects antibiotic therapy. If the same dose of penicillin or ampicillin is administered to both nonpregnant and pregnant women, lower serum levels are attained during pregnancy because of the distribution of the drug in a larger intravascular volume.
Transplacental passage of penicillin is by simple diffusion. The free-circulating portion of the antibiotic crosses the placenta, resulting in a lower maternal serum level of the unbound portion of the drug. Administration of penicillins with high protein-binding (e.g., oxacillin, cloxacillin [Cloxapen], dicloxacillin [Pathocil], and nafcillin [Unipen]) to a pregnant woman leads to lower fetal tissue and amniotic fluid levels than the administration of poorly bound penicillins (e.g., penicillin G and ampicillin).
The antibiotic is ultimately excreted in the fetal urine and thus into the amniotic fluid. The delay in appearance of different types of penicillins in the amniotic fluid depends primarily on the rate of transplacental diffusion, the amount of protein-binding in fetal serum, and the adequacy of fetal enzymatic and renal function. A time delay may occur before effective levels of the antibiotic appear in the amniotic fluid.
Cephalosporins
In a large case-control study, Czeizel and associates128 found no teratogenic risks from cephalosporin use. Maternal serum levels of cephalosporins are lower than in nonpregnant patients receiving equivalent dosages because of a shorter half-life in pregnancy, an increased volume of distribution, and increased renal elimination. They readily cross the placenta to the fetal bloodstream and, ultimately, the amniotic fluid.
Sulfonamides
Among 1455 human infants exposed to sulfonamides during the first trimester, no teratogenic effects were noted.99 However, the administration of sulfonamides should be avoided in women deficient in glucose-6-phosphate dehydrogenase (G6PD), because a dose-related toxic reaction may occur resulting in red cell hemolysis.
Sulfonamides cause no known damage to the fetus in utero, because the fetus can clear free-bilirubin through the placenta. These drugs theoretically might have deleterious effects if present in the blood of the neonate after birth, however. Sulfonamides compete with bilirubin for binding sites on albumin, thus raising the levels of free bilirubin in the serum and increasing the risk of hyperbilirubinemia in the neonate. Although this toxicity occurs with direct administration to the neonate, kernicterus in the newborn after in utero exposure has not been reported.129 The sulfonamides are easily absorbed orally and readily cross the placenta, achieving fetal plasma levels 50–90% of those attained in the mother.
Sulfasalazine (Azulfidine) is used for treatment of ulcerative colitis and Crohn's disease because of its relatively poor oral absorption. However, it crosses the placenta, leading to fetal drug concentrations approximately the same as those of the mother, although both are low. Neither kernicterus nor severe neonatal jaundice have been reported after maternal use of sulfasalazine, even when the drug was administered up to the time of delivery.130
FOLIC ACID ANTAGONISTS
Trimethoprim often is administered with sulfa to treat urinary tract infections. Two small trials including 131 women failed to show any increased risk of birth defects after first-trimester exposure with trimethoprim.131, 132 However, one unpublished study of 2296 Michigan Medicaid recipients suggests an increased risk of cardiovascular defects after exposure in the first trimester.133 In a retrospective study of trimethoprim with sulfamethoxazole, the odds ratio for birth defects was 2:3.134 Supplementation with folic acid might reduce this risk.
Folic acid antagonists, which include trimethoprim, may increase the risk not only of neural-tube defects but also of cardiovascular defects, oral clefts, and urinary tract defects.135
Nitrofurantoin
Nitrofurantoin (Macrodantin) is used in the treatment of acute, uncomplicated, lower urinary tract infections and for long-term suppression in patients with chronic bacteriuria. Nitrofurantoin is capable of inducing hemolytic anemia in patients deficient in G6PD. However, hemolytic anemia in the newborn as a result of in utero exposure to nitrofurantoin has not been reported.
No reports have linked the use of nitrofurantoin with congenital defects. In the Collaborative Perinatal Project,99 590 infants were exposed84 during the first trimester, with no increased risk of adverse effects. Other studies have confirmed these findings.136, 137
Nitrofurantoin absorption from the gastrointestinal tract varies with the form administered. The macrocrystalline form is absorbed more slowly than the crystalline and is associated with less gastrointestinal intolerance. Because of rapid elimination, the serum half-life is 20–60 minutes. Therapeutic serum levels are not achieved; therefore, this drug is not indicated when there is a possibility of bacteremia. Approximately one third of an oral dose appears in the active form in the urine.
Tetracyclines
The tetracyclines readily cross the placenta and are firmly bound by chelation to calcium in developing bone and tooth structures. This produces brown discoloration of the deciduous teeth, hypoplasia of the enamel, and inhibition of bone growth.138 The staining of the teeth takes place in the second or third trimesters of pregnancy, whereas bone incorporation can occur earlier. Depression of skeletal growth was particularly common among premature infants treated with tetracycline. Hepatotoxicity has been reported in pregnant women treated with tetracyclines in large doses, usually with intravenous administration for pyelonephritis.
First-trimester exposure to tetracyclines has not been found to have any teratogenic risk in 341 women in the Collaborative Perinatal Project99 or in 174 women in another study.139 First-trimester exposure to doxycycline (Vibramycin) is not known to have any risk.140 Alternate antibiotics currently are recommended during pregnancy.
Aminoglycosides
Streptomycin and kanamycin have been associated with congenital deafness in the offspring of mothers who used these drugs during pregnancy. Ototoxicity was reported with doses as low 1 g streptomycin biweekly for 8 weeks during the first trimester.141 Of 391 mothers who had received 50 mg/kg of kanamycin for prolonged periods during pregnancy, nine children (2.3%) were found to have hearing loss.142
Nephrotoxicity may be greater when aminoglycosides are combined with cephalosporins. Neuromuscular blockade may be potentiated by the combined use of aminoglycosides and curariform drugs; therefore, the dosages should be reduced appropriately. Potentiation of magnesium sulfate-induced neuromuscular weakness also has been reported in a neonate exposed to magnesium sulfate and gentamicin (Garamycin).143
No known teratogenic effect other than ototoxicity has been associated with the use of aminoglycosides in the first trimester. In 135 infants exposed to streptomycin in the Collaborative Perinatal Project,99 no teratogenic effects were observed. In a group of 1619 newborns whose mothers were treated for tuberculosis with multiple drugs, including streptomycin, the incidence of congenital defects was the same as in a healthy control group.144
Aminoglycosides are poorly absorbed after oral administration and are rapidly excreted by the normal kidney. Because the rate of clearance is related to the glomerular filtration rate, dosage must be reduced in the face of abnormal renal function. The serum aminoglycoside levels usually are lower in pregnant than in nonpregnant patients receiving equivalent doses because of more rapid elimination.145 Thus, levels must be monitored to prevent subtherapeutic dosing.
Once-per-day dosing with gentamicin at 4 mg/kg, in treatment of puerperal infections, increases efficacy and decreases toxicity and cost.146
Antituberculosis Drugs
There is no evidence of any teratogenic effect of isoniazid, para-aminosalicylic acid, rifampin (Rifadin), or ethambutol (Myambutol). Because of increased risk for hepatic toxicity in pregnancy, the American Thoracic Society recommends delaying preventative treatment with isoniazid for asymptomatic purified protein derivative conversion until after pregnancy.147
Macrolides
No teratogenic risk of erythromycin has been reported. In 79 patients in the Collaborative Perinatal Project99 and 260 in another study,139 no increase in birth defects was noted.
Erythromycin is not consistently absorbed from the gastrointestinal tract of pregnant women, and the transplacental passage is unpredictable. Both maternal and fetal plasma levels achieved after the administration of the drug in pregnancy are low and vary considerably, with fetal plasma concentrations being 5–20% of those in maternal plasma.148 Thus, some authors recommend that penicillin be administered to every newborn whose mother received erythromycin for the treatment of syphilis.149 Fetal tissue levels increase after multiple doses.148 The usual oral dose is 250–500 mg every 6 hours, but the higher dose may not be well-tolerated in pregnant women who are susceptible to gastrointestinal symptoms.
Of 122 first-trimester exposures to clarithromycin (Biaxin), there was no significant risk of birth defects.150
Of 647 infants exposed to clindamycin (Cleocin) in the first trimester, no increased risk of birth defects was noted.151
Clindamycin is nearly completely absorbed after oral administration; a small percentage is absorbed after topical application. The drug crosses the placenta, achieving maximum cord serum levels of approximately 50% of the maternal serum.148
Clindamycin is 90% bound to serum protein, and fetal tissue levels increase after multiple dosing. Maternal serum levels after dosing at various stages of pregnancy are similar to those of nonpregnant patients.
Quinolones
The quinolones (e.g., ciprofloxacin [Cipro], norfloxacin [Noroxin]) have a high affinity for bone tissue and cartilage and may cause arthralgia in children. However, no malformations or musculoskeletal problems were noted in 38 infants exposed in utero in the first trimester.152 Of 132 newborns exposed in the first trimester in the Michigan Medicaid data, no increased risk for birth defects was noted.153 The manufacturer recommends against use in pregnancy and in children.
Metronidazole
Studies have failed to show any increase in the incidence of congenital defects among the newborns of mothers treated with metronidazole (Flagyl) during early or late gestation. In a study of 1387 prescriptions filled, no risk of birth defects could be determined.154 A recent meta-analysis confirmed no teratogenic risk.155
ANTIRETROVIRALS
In a prospective cohort study, children exposed to Zidovudine (AZT) in the perinatal period through Pediatric AIDS Clinical Trials Group Protocol 076 were studied up to a median age of 4.2 years. No adverse effects were observed in these children.156 Combination antiretroviral therapy when used in pregnancy has not been associated with major infant toxicity even when the therapy was initiated in the first trimester.157
Prenatal exposure has been reported in all three trimesters of pregnancy to almost all of the antiretroviral drugs alone or in combination with the exception of zalcitabine and delavirdine. Exposure to the latter two drugs has been reported in the first and second trimesters. No teratogenicity has been reported.158
Lindane
After application of lindane (Kwell) to the skin, approximately 10% of the dose used can be recovered in the urine. Toxicity in humans after use of topical 1% lindane has been observed almost exclusively after misuse and overexposure to the agent. Although no evidence of specific fetal damage is attributable to lindane, the agent is a potent neurotoxin, and its use during pregnancy should be limited. Pregnant women should be cautioned about shampooing their children's hair, because absorption could easily occur across the skin of the hands of the mother. An alternate drug for lice usually is recommended, such as pyrethrins with piperonyl butoxide (RID).
ANTIFUNGAL AGENTS
Nystatin (Mycostatin) is poorly absorbed from intact skin and mucous membranes, and topical use has not been associated with teratogenesis.128
The imidazoles are absorbed in only small amounts from the vagina. Clotrimazole (Lotrimin) or miconazole (Monistat) in pregnancy is not known to be associated with congenital malformations. However, in one study, a statistically significantly increased risk of first-trimester abortion was noted after use of these drugs, but these findings were considered not to be definitive evidence of risk.159 Of 2624 newborns exposed in the first trimester in the Michigan Medicaid data, there was no increased risk of anomalies.160
An increased risk of limb deformities was reported in fetuses exposed to 400–800 mg per day of fluconazole in the first trimester.161 In 460 who received a single 150-mg dose of fluconazole, no increased risk of defects was observed.162, 163
ANTIASTHMASTICS
Theophylline and Aminophylline
Both theophylline (Theo-Dur, Slo-Bid) and aminophylline are safe for the treatment of asthma in pregnancy. No evidence of teratogenic risk was found in 76 exposures in the Collaborative Perinatal Project.99 Because of increased renal clearance in pregnancy, dosages might need to be increased.
Epinephrine (Adrenalin)
Minor malformations have been reported with sympathomimetic amines as a group in 3082 exposures in the first trimester, usually in commercial preparations used to treat upper respiratory infections.99
Terbutaline
Terbutaline (Brethine) has been widely used in the treatment of preterm labor. It has a more rapid onset and a longer duration of action than epinephrine and is preferred for asthma in the pregnant patient. No risk of birth defects has been reported. Long-term use has been associated with an increased risk of glucose intolerance.164
Cromolyn Sodium
Cromolyn sodium (Intal) may be administered in pregnancy; the systemic absorption is minimal. Teratogenicity has not been reported in humans.
Isoproterenol and Metaproterenol
When isoproterenol (Isuprel) and metaproterenol (Alupent) are administered as topical aerosols for the treatment of asthma, the total dose absorbed usually is not significant. With oral or intravenous doses, however, the cardiovascular effects of the agents may result in decreased uterine blood flow. For this reason, they should be used with caution. No teratogenicity has been reported.99
Corticosteroids
All steroids cross the placenta to some degree, but prednisone (Deltasone) and prednisolone are inactivated by the placenta. When prednisone or prednisolone are maternally administered, the concentration of active compound in the fetus is less than 10% of that in the mother. Therefore, these agents are the drugs of choice for treating medical diseases such as asthma. Inhaled corticosteroids also are effective therapy, and little of the drug is absorbed. When steroid effects are desired in the fetus, for example, to accelerate lung maturity, betamethasone (Celestone) and dexamethasone (Decadron) are preferred, because these are minimally inactivated by the placenta. However, a five-fold increased risk for cleft lip with or without cleft palate in the infant has been reported after exposure to steroids in the first trimester.105,106
Iodide
Iodide, such as is found in a saturated solution of potassium iodide expectorant, crosses the placenta and may produce a large fetal goiter, enough to produce respiratory obstruction in the newborn.69 Before a patient is advised to use a cough medicine, she should be sure to ascertain that it does not contain iodide.
CARDIOVASCULAR AND ANTIHYPERTENSIVE AGENTS
Digoxin
In 52 exposures, no teratogenicity of digoxin (Lanoxin) was noted.99 Blood levels should be monitored in pregnancy to ensure adequate therapeutic maternal levels.
Digoxin-like immunoreactive substances may be mistaken in assays for fetal concentrations of digoxin. In one study of fetuses with cardiac anomalies,165 there was no difference in the immunoreactive digoxin levels whether or not the mother had received digoxin. In hydropic fetuses, digoxin may not easily cross the placenta.166
Alpha-methyldopa (Aldomet) has been widely used for the treatment of chronic hypertension in pregnancy. Although postural hypotension may occur, no unusual fetal effects have been noted. Hydralazine (Apresoline) also has had widespread use in pregnancy, and no teratogenic effect has been observed.
β-Adrenergic Blocking Agents
Propranolol (Inderal) is a β-adrenergic blocking agent in widespread use for a variety of indications. No evidence of teratogenicity has been found. Bradycardia has been reported in the newborn as a direct effect of a dose of the drug administered to the mother within 2 hours of delivery of the infant.167
Several studies of propranolol use in pregnancy show an increased risk of intrauterine growth restriction or at least a skewing of the birth weight distribution toward the lower range.168 Ultrasound monitoring of patients on this drug is prudent.
Atenolol (Tenormin) is a cardioselective β-blocker. In a large analysis of published trials involving β-blocker therapy, there was little information on teratogenicity for the multiple agents reported including atenolol, labetalol, metoprolol, oxprenolol, pindolol and propranolol.169 Atenolol was associated with lower birth weight and a trend toward more preterm delivery compared with other antihypertensive drugs or no therapy. These effects were more pronounced when the drug was administered earlier in pregnancy and for long durations.170 In one study, treatment of hypertension (mostly with atenolol) reduced the risk of severe hypertension and preterm labor. These authors felt that the therapy had to be adjusted to avoid an excessive decrease in cardiac output or an increase in vascular resistance as these were associated with reduced fetal growth and not the atenolol per se.171 The same group showed that atenolol prevented preeclampsia in a double-blinded, randomized, placebo-controlled trial but did result in infants with birth weights of 440 g less than the placebo group.
Angiotensin-Converting Enzyme Inhibitors
Angiotenin-converting enzyme (ACE) inhibitors drugs do not appear to be teratogenic in the first trimester.172 ACE inhibitors (e.g., enalapril [Vasotec], captopril [Capoten]) can cause fetal renal tubular dysplasia in the second and third trimesters, leading to oligohydramnios, fetal limb contractures, craniofacial deformities, and hypoplastic lung development.173 Fetal skull ossification defects also have been described.174 For these reasons, ACE inhibitors should be stopped when pregnancy is diagnosed, and pregnant women should receive other agents.
DRUGS FOR OVULATION INDUCTION
In more than 2000 exposures, no evidence of teratogenic risk of clomiphene (Clomid) has been noted,175 and the percentage of spontaneous abortions is close to the expected rate. Although infants often are exposed to bromocriptine (Parlodel) in early pregnancy, no teratogenic effects have been observed in more than 1400 pregnancies.176
INADVERTANT EXPOSURE TO SEX STEROIDS AND CONTRACEPTIVE AGENTS
Estrogens and Progestins
Oral contraceptives and progestins administered in the first trimester have been blamed for a variety of birth defects, but studies have not confirmed any teratogenic risk for these drugs.177
One study of 2754 infants born to mothers after bleeding in the first trimester suggests no increased risk associated with first-trimester exposure to progestogens.178 A meta-analysis of first-trimester sex hormone exposure reveals no association between exposure and fetal genital malformations.179
Androgenic Steroids
Androgens may masculinize a developing female fetus. Progestational agents, most often the synthetic testosterone derivatives, may cause clitoromegaly and labial fusion if administered before 13 weeks of pregnancy.180 Danazol (Danocrine) has been reported to produce mild clitoral enlargement and labial fusion when administered inadvertently for the first 10–12 weeks after conception.181
Spermicides
A reported increased risk for abnormal offspring in mothers who had used spermicides for contraception has not been confirmed. In the Collaborative Perinatal Project data,182 462 women reported spermicide use in the first 4 lunar months, with 438 also describing use during the month preceding the last menstrual period. The exposed women had 23 infants with anomalies (5%) compared with 4.5% in the nonexposed controls, which is not a significant difference.
In the study of Mills and coworkers,183 the malformation rate in women using spermicides after their last menstrual period was 4.8 in 1000 compared with 6.4 in 1000 in the controls, which is not significantly different. The risks for preterm delivery, a low-birth-weight infant, and spontaneous abortion were no higher in the spermicide-exposed group. The study of Linn and colleagues184 of 12,440 women found no relationship between contraceptive method and the occurrence of malformations.
SOCIAL DRUG EXPOSURE
Smoking
Smoking has been associated with a four-fold increase in small size for gestational age and an increased prematurity rate.185 The spontaneous abortion rate is up to twice that of nonsmokers. Abortions associated with maternal smoking tend to have a higher percentage of normal karyotypes and occur later than those with chromosomal aberrations.186 The higher perinatal mortality rate associated with smoking is attributable to an increased risk for abruptio placentae, placenta previa, premature rupture of membranes, and intrauterine growth restriction. The risks for complications and of the associated perinatal loss increase with the number of cigarettes smoked. Discontinuation of smoking during pregnancy can reduce the risk of pregnancy complications and perinatal mortality, especially in women at high risk for other reasons.187 Maternal passive smoking also was associated with a two-fold risk for low birth weight at term in one study.188
There also is a positive association between smoking and sudden infant death syndrome and increased respiratory illnesses in children. In such reports, it is not possible to distinguish between apparent effects of maternal smoking during pregnancy and smoking after pregnancy, but both may play a role.
Smoking Cessation During Pregnancy
Tobacco smoke contains nicotine, carbon monoxide, and many other compounds. Although nicotine is the mechanism of addiction to cigarettes, other chemicals may contribute to adverse pregnancy outcome. For example, carbon monoxide decreases oxygen delivery to the fetus, whereas nicotine decreases uterine blood flow.
Nicotine withdrawal may first be attempted with nicotine fading, switching to brands of cigarettes with progressively less nicotine over a 3-week period. Exercise also may improve quitting success rates.189 Nicotine medications are indicated for patients with nicotine dependence. This is defined as smoking more than one pack per day, smoking within 30 minutes of getting up in the morning, or having previous withdrawal symptoms.190 Nicotine medications are available as patches, gum, or inhalers. Although the propriety of prescribing nicotine during pregnancy may be questioned, if the patient can stop smoking, she eliminates many other toxins, including carbon monoxide, and nicotine blood levels are similar to that of smokers.191 There are no studies of bupropion (Zyban) in human pregnancy for smoking cessation.
Alcohol
Fetal alcohol syndrome (FAS) has been reported in the offspring of mothers with alcoholism and includes the features of gross physical retardation with onset prenatally and continuing after birth.192
The Fetal Alcohol Study Group of the Research Society on Alcoholism has strict criteria for the diagnosis of FAS.193 At least one characteristic from each of the following three categories has to be present for a valid diagnosis of the syndrome: (1) growth retardation before or after birth; (2) facial anomalies, including small palpebral fissures, indistinct or absent philtrum, epicanthic folds, flattened nasal bridge, short length of nose, thin upper lip, low-set unparallel ears, and retarded midfacial development; and (3) central nervous system dysfunction including microcephaly, varying degrees of mental retardation, or other evidence of abnormal neurobehavioral development, such as attention deficit disorder with hyperactivity.
None of these features is individually pathognomonic for fetal alcohol exposure. Confirmatory evidence for this diagnosis is a history of heavy maternal drinking during pregnancy.
In the study of Jones and coworkers,194 23 women with long-term alcoholism were matched with 46 controls, and the pregnancy outcomes of the two groups were compared. Among the alcoholic mothers, perinatal deaths were approximately eight-times more frequent. Growth restriction, microcephaly, and IQ less than 80 were considerably more frequent than among the controls. Overall outcome was abnormal in 43% of the offspring of the alcoholic mothers compared with 2% of the controls.
Ouellette and associates195 addressed the risks of smaller amounts of alcohol. Nine percent of infants of abstinent or rare drinkers and 14% of infants of moderate drinkers were abnormal, which is not a significant difference. In heavy drinkers (average daily intake of 3 oz of 100-proof liquor or more), 32% of the infants had anomalies. Overall, including anomalies, growth restriction, and an abnormal neurologic examination, 71% of the children of heavy drinkers were affected, which is twice the frequency of abnormality found in the moderate and rarely drinking groups. In this study, an increased frequency of abnormality was not found until 45 mL of ethanol (equivalent to three drinks) daily were exceeded. The study of Mills and Graubard196 also shows that total malformation rates were not significantly higher among offspring of women who had an average of less than one drink per day (77.3/1000) or one to two drinks per day (83.2/1000) than among nondrinkers (78.1/1000). Genitourinary malformations increased with increasing alcohol consumption, however, so the possibility remains that for some malformations, no safe drinking level exists.
Heavy drinking remains a major risk to the fetus, and reduction even during mid pregnancy can benefit the infant. An occasional drink during pregnancy has no known risk, but no level of drinking is known to be safe.
Sokol and coworkers197 address history taking for prenatal detection of risk drinking. Four questions help differentiate patients drinking sufficiently to potentially damage the fetus (Table 2). The patient is considered at risk if more than two drinks are required to make her feel “high.” The probability of “risk drinking” increases to 63% for those responding positively to all four questions.
Table 2. T-ACE Questions for Identifying Women Drinking Sufficiently to Potentially Damage the Fetus
| T | How many drinks does it take to make you feel high (how many drinks can you hold, i.e., what is your tolerance?) |
| A | Have people annoyed you by criticizing your drinking? |
| C | Have you felt you should decrease your drinking? |
| E | Have you ever had to drink first thing in the morning to steady your nerves or to get rid of a hangover? (eye-opener) |
Two points are scored as a positive answer to the tolerance question and one point each for the other three. A score of 2 or more correctly identified 69% of risk drinkers. (Sokol RJ, Martier SS, Ager JW: The T-ACE questions: Practical prenatal detection of risk-drinking. Am J Obstet Gynecol 160:863, 1989.)
Caffeine
There is no evidence of teratogenic effects of caffeine in humans. The Collaborative Perinatal Project99 showed no increased incidence of congenital defects in 5773 women using caffeine in pregnancy, usually in a fixed-dose analgesic medication. Controversy still exists concerning the association between heavy ingestion of caffeine and increased pregnancy complications. Early studies suggest that the intake of more than seven to eight cups of coffee per day (one cup of coffee contains approximately 100 mg of caffeine) was associated with low-birth-weight infants, spontaneous abortions, prematurity, and stillbirths.198 However, these studies were not controlled for the concomitant use of tobacco and alcohol. In one report controlled for smoking, other habits, demographic characteristics, and medical history, no relationship was found between either low birth weight or short gestation and heavy coffee consumption.199 Also, there was no excess of malformations among coffee drinkers. When pregnant women consumed more than 300 mg per day caffeine, one study suggests an increase in term low-birth-weight infants200 weighing than 2500 g at greater than 36 weeks.
Concomitant consumption of caffeine with cigarette smoking may increase the risk of low birth weight201. Maternal coffee intake decreases iron absorption and may contribute to maternal anemia.202
Two other studies show conflicting results. One retrospective investigation reporting a higher risk of fetal loss was biased by ascertainment of the patients at the time of fetal loss, because these patients typically have less nausea and would be expected to drink more coffee.203 A prospective cohort study found no evidence that moderate caffeine use increased the risk of spontaneous abortion or growth retardation.204 Measurement of serum paraxanthine, a caffeine metabolite, reveals that only high levels are associated with spontaneous abortions.205
Aspartame
The major metabolite of aspartame (NutraSweet) is phenylalanine,206 which is concentrated in the fetus by active placental transport. Sustained high blood levels of phenylalanine in the fetus, as seen in maternal phenylketonuria, are associated with mental retardation in the infant. Within the usual range of aspartame ingestion, peak phenylalanine levels do not exceed normal postprandial levels, and even with high doses, phenylalanine concentrations still are far below those associated with mental retardation. These responses also have been studied in women known to be carriers of phenylketonuria, and the levels are still normal. Thus, it seems unlikely that use of aspartame in pregnancy would cause any fetal toxicity.
Narcotics
The goal of methadone maintenance is to bring the patient to a level of approximately 20 to 40 mg per day. The dose should be individualized at a level sufficient to minimize the use of supplemental illicit drugs, because they represent a greater risk to the fetus than do the higher doses of methadone required by some patients. Manipulation of the dose in women maintained on methadone should be avoided in the last trimester because of an association with increased fetal complications and in utero deaths attributed to fetal withdrawal in utero.207 Because management of narcotic addiction during pregnancy requires a host of social, nutritional, educational, and psychiatric interventions, these patients are best managed in specialized programs.
The infant of a woman addicted to narcotics is at increased risk for abortion, prematurity, and growth restriction. Withdrawal should be watched for carefully in the neonatal period.
Marijuana
No teratogenic effect of marijuana has been documented. In a prospective study of 35 pregnancies,208 infants born to marijuana users exhibited significantly more meconium staining (57 vs. 25% in nonusers). However, users tended to come from lower socioeconomic backgrounds. Most adverse outcomes of pregnancy were too infrequent to permit reliable comparisons between the groups. Marijuana users had an increased incidence of precipitate labor (less than 3 hours total): 29% compared with 3% in the control group.208
In another population in which the users and nonusers of marijuana were similar in general health, ethnic background, nutritional habits, and use of tobacco, these differences were not confirmed (19 patients in each group).209 In this same small group of patients, average use of marijuana six or more times per week during pregnancy was associated with a reduction of 0.8 weeks in the length of gestation, although no reduction in birth weight was noted. One study suggests a mean 73-g decrease in birth weight associated with marijuana use when urine assays were performed rather than relying on self-reporting.210
Cocaine
A serious difficulty in determining the effects of cocaine on the infant is the frequent presence of many confounding variables in the population using cocaine. These mothers often abuse other drugs, smoke, have poor nutrition, fail to seek prenatal care, and live under poor socioeconomic conditions. These factors are difficult to take into account in comparison groups. Another difficulty is the choice of outcome measures for infants exposed. The neural systems likely to be affected by cocaine are involved in neurologic and behavioral functions that are not easily quantitated by standard infant development tests.
Cocaine-using women have a higher rate of spontaneous abortion than controls.211 Three studies suggest an increased risk for congenital anomalies after first-trimester cocaine use,212, 213, 214 most frequently cardiac and central nervous system. In the study of Bingol and colleagues,213 the malformation rate was 10% in cocaine users, 4.5% in polydrug users, and 2% in controls. MacGregor and associates214 report a 6% anomaly rate compared with 1% for controls.
Cocaine is a central nervous system stimulant and has local anesthetic and marked vasoconstrictive effects. Abruptio placentae has been reported to occur immediately after nasal or intravenous administration.211 Several studies also note increased stillbirths, preterm labor, premature birth, and small-for-gestational age infants with cocaine use.210, 211, 212, 213, 214, 215, 216
The impairment of intrauterine brain growth as manifested by microcephaly is the most common brain abnormality in infants exposed to cocaine in utero.217 In one study, 16% of newborns had microcephaly compared with 6% of controls.218 Somatic growth also is impaired, so the growth restriction may be symmetric or characterized by a relatively low head circumference-to-abdominal circumference ratio.219 Multiple other neurologic problems have been reported after cocaine exposure, as well as dysmorphic features and neurobehavioral abnormalities.216
Aside from causing congenital anomalies in the first trimester, cocaine has been reported to cause fetal disruption,220 presumably from interruption of blood flow to various organs. Bowel infarction has been noted, with unusual ileal atresia and bowel perforation. Limb infarction has resulted in missing fingers in a distribution different from the usual congenital limb anomalies. Central nervous system bleeding in utero may result in porencephalic cysts.
CONCLUSION
Patients should be educated about methods other than drugs to cope with tension, aches and pains, and viral illnesses during pregnancy. The risk-to-benefit ratio should justify the use of any drug and the minimum effective dose should be used. Patients should be educated about risks of social drug exposure. Because long-term effects of drugs in utero may not be revealed for many years, caution with regard to any drug use in pregnancy is warranted.
REFERENCES
Knapp K, Lenz W, Nowack E: Multiple congenital abnormalities. Lancet 2:725, 1962 |
|
Wilson JG: Environment and Birth Defects. New York, Academic Press, 1973 |
|
Wilson JG: Differentiation and the reaction of rat embryos to radiation. J Cell Comp Physiol 43:Suppl 1:11, 1954 |
|
Fraser FC, Fainstat TD: Production of congenital defects in the offspring of pregnant mice treated with cortisone. Pediatrics 8:527, 1951 |
|
Jacobson BD: Hazards of norethindrone therapy during pregnancy. Am J Obstet Gynecol 84:962, 1962 |
|
Phelan MC, Pollock JM, Nance WE: Discordant expression of fetal hydantoin syndrome in heteropaternal dizygotic twins. N Engl J Med 307:99, 1982 |
|
Vessell ES: Genetic and environmental factors affecting drug metabolism in man. Proceedings of the 4th International Congress on Human Genetics Paris, International Congress Series 250, 391, 1972 |
|
Peer LA, Bryan WH, Strean LP et al: Induction of cleft palate in mice by cortisone and its reduction by vitamins. J Int Coll Surg 30:249, 1958 |
|
Kimmel CA, Wilson JG, Schumacher HJ: Studies on metabolism and identification of the causative agent in aspirin teratogenesis in the rat. Teratology 4:15, 1971 |
|
Kalter M: Interplay of intrinsic and extrinsic factors. In: Wilson JG, Warkany J (eds): Teratology: Principles and Techniques, Chicago. University of Illinois Press, 57, 1965 |
|
Saxen L: Defective regulatory mechanisms in teratogenesis. Int J Gynaecol Obstet 8:798, 1970 |
|
Simpson JL: Relationship between congenital anomalies and contraception. Adv Contracep 1:3, 1985 |
|
Sever LE, Mortenson ML: Teratology and the epidemiology of birth defects. In: Gabbe SG, Niebyl JR, Simpson JL (eds): Obstetrics: Normal and Problem Pregnancies. New York, Churchill-Livingstone, 185–214, 1996 |
|
Wilson JG, Fraser FC (eds): Handbook of Teratology. New York, Plenum, 1979 |
|
Teratology Society Public Affairs Committee. FDA Classification of drugs for teratogenic risk Teratol 49:446, 1994 |
|
Doering PL, Boothby LA, Cheok M: Review of pregnancy labeling of prescription drugs: Is the current system adequate to inform of risks. Am J Obstet Gynecol 187:333, 2002 |
|
Lammer EJ, Chen DT, Hoar RM et al: Retinoic acid embryopathy. N Engl J Med 313:837, 1985 |
|
Adams J: High incidence of intellectual deficits in 5-year-old children exposed to isotretinoin “in utero”. Teratology 41:614, 1990 |
|
Dai WS, Hsu M-A, Itri L: Safety of pregnancy after discontinuation of isotretinoin. Arch Dermatol 125:362, 1989 |
|
Jick SS, Terris BZ, Jick H: First trimester topical tretinoin and congenital disorders. Lancet 341:1181, 1993 |
|
Geiger JM, Baudin M, Saurat JH: Teratogenic risk with etritinate and acitretin treatment. Dermatology 189:109, 1994 |
|
Rothman KJ, Moore LL, Singer MR et al: Teratogenicity of high vitamin A intake. N Engl J Med 333:1369, 1995 |
|
Mills JL, Simpson JL, Cunningham GC et al: Vitamin A and birth defects. Am J Obstet Gynecol 177:31, 1997 |
|
Kozlowski RD, Steinbrunner JV, MacKenzie AH et al: Outcome of first-trimester exposure to low-dose methotrexate in eight patients with rheumatic disease. Am J Med 88:589, 1990 |
|
Armenti VT, Ahlswede BA, Ahlswede JR et al: National transplantation pregnancy registry: Analysis of outcome/risks of 394 pregnancies in kidney transplant recipients. Transplant Proc 26:2535, 1994 |
|
Polifka JE, Friedman JM: Teratogen update: Azathioprine and 6-mercaptopurine. Teratol 65:240, 2002 |
|
Armenti VT, Moritz MJ, Davison JM: Drug safety issues in pregnancy following transplantation and immunosuppression: Effects and outcomes. Drug Saf 19:219, 1998 |
|
Takahashi N, Nishida H, Hoshi J: Severe B cell depletion in newborns from renal transplant mothers taking immunosuppressive agents. Transplantation 57:1617, 1994 |
|
Briggs GC, Freeman RK, Yaffe SJ: Drugs in Pregnancy and Lactation. 6th ed.. Baltimore, Williams & Wilkins, 339, 2002 |
|
Wolfe MS, Cordero JF: Safety of chloroquine in chemosuppression of malaria during pregnancy. Br Med J 290:1466, 1985 |
|
Hart CW, Naunton RF: The ototoxicity of chloroquine phosphate. Arch Otolaryngol 80:407, 1964 |
|
Levy M, Buskila D, Gladman DD et al: Pregnancy outcome following first trimester exposure to chloroquine. Am J Perinatol 8:174, 1991 |
|
Zemlickis D, Lishner M, Degendorfer P et al: Fetal outcome after in utero exposure to cancer chemotherapy. Arch Intern Med 152:573, 1992 |
|
Robert E, Guibaud P: Maternal valproic acid and congenital neural tube defects. Lancet 2:937, 1982 |
|
Rosa FW: Spina bifida in infants of women treated with carbamazepine during pregnancy. N Engl J Med 324:674, 1991 |
|
Holmes LB, Harvey EA, Coull BA et al: The teratogenicity of anticonvulsant drugs. N Engl J Med 344:1132, 2001 |
|
Dansky LV, Rosenblatt DS, Andermann E: Mechanisms of teratogenesis: Folic acid and antiepileptic therapy. Neurology 42:32, 1992 |
|
Centers for Disease Control: Recommendations for the use of folic acid to reduce the number of cases of spina bifida and other neural tube defects. MMWR Morb Mortal Wkly Rep 41:1, 1992 |
|
Biale Y, Lewenthal H: Effect of folic acid supplementation on congenital malformations due to anticonvulsive drugs. Eur J Obstet Gynecol Reprod Biol 18:211, 1984 |
|
Hanson JW, Smith DW: The fetal hydantoin syndrome. J Pediatr 87:285, 1975 |
|
Gaily E, Granstrom M-L, Hiilesmaa V et al: Minor anomalies in offspring of epileptic mothers. J Pediatr 112:520, 1988 |
|
Jones KL, Lacro RV, Johnson KA et al: Pattern of malformations in the children of women treated with carbamazepine during pregnancy. N Engl J Med 320:1661, 1989 |
|
Strickler SM, Miller MA, Andermann E et al: Genetic predisposition to phenytoin-induced birth defects. Lancet 2:746, 1985 |
|
Buehler BA, Delimont D, VanWaes M et al: Prenatal prediction of risk of the fetal hydantoin syndrome. N Engl J Med 322:1567, 1990 |
|
Van der Pol MC, Hadders-Algra M, Huisjes JH et al: Antiepileptic medication in pregnancy: Late effects on the children's central nervous system development. Am J Obstet Gynecol 164:121, 1991 |
|
Scolnik D, Nulman I, Rovet J et al: Neurodevelopment of children exposed in utero to phenytoin and carbamazepine monotherapy. JAMA 271:767, 1994 |
|
Reinisch JM, Sanders SA, Mortensen EL et al: In utero exposure to phenobarbital and intelligence deficits in adult men. JAMA 274:1518, 1995 |
|
Reiff-Eldridge R, Heffner CR, Ephross SA et al: Monitoring pregnancy outcomes after prenatal drug exposure through prospective pregnancy registries: A pharmaceutical company commitment. Am J Obstet Gynecol 182:159, 2000 |
|
Briggs GG, Freeman RK, Yaffe SJ: Drugs in Pregnancy and Lactation. 6th ed.. Baltimore, Williams & Wilkins, 766–769, 2002 |
|
Tennis P, Eldridge RR: International Lamotrigine Pregnancy Registry Scientific Advisory Committee. Preliminary results on pregnancy outcomes in women using lamotrigine Epilepsia 43:1161, 2002 |
|
Callaghan N, Garrett A, Goggin T: Withdrawal of anticonvulsant drugs in patients free of seizures for two years. N Engl J Med 318:942, 1988 |
|
Deblay MF, Vert P, Andre M et al: Transplacental vitamin K prevents haemorrhagic disease of infant of epileptic mother. Lancet 1:1247, 1982 |
|
Haddow JE, Palomaki GE, Allan WC et al: Maternal thyroid deficiency during pregnancy and subsequent neuropsychological development of the child. N Engl J Med 341:549, 1999 |
|
Hall JG, Pauli RM, Wilson KM: Maternal and fetal sequelae of anticoagulation during pregnancy. Am J Med 68:122, 1980 |
|
Sbarouni E, Oakley CM: Outcome of pregnancy in women with valve prostheses. Br Heart J 71:196, 1994 |
|
deSwiet M, Ward PD, Fidler J et al: Prolonged heparin therapy in pregnancy causes bone demineralization. Br J Obstet Gynaecol 90:1129, 1983 |
|
Barbour LA, Kick SD, Steiner JF et al: A prospective study of heparin-induced osteoporosis in pregnancy using bone densitometry. Am J Obstet Gynecol 170:862, 1994 |
|
Dahlman TC: Osteoporotic fractures and the recurrence of thromboembolism during pregnancy and the puerperium in 184 women undergoing thromboprophylaxis with heparin. Am J Obstet Gynecol 168:1265, 1993 |
|
Nelson-Piercy C, Letsky EA, deSwiet M: Low-molecular-weight heparin for obstetric thromboprophylaxis: Experience of sixty-nine pregnancies in sixty-one women at high risk. Am J Obstet Gynecol 176:1062, 1997 |
|
Casele HL, Laifer SA, Woelkers DA et al: Changes in the pharmacokinetics of the low-molecular-weight heparin enoxaparin sodium during pregnancy. Am J Obstet Gynecol 181:1113, 1999 |
|
Ginsberg JS, Hirsh J: Use of antithrombotic agents during pregnancy. Chest 114:524S, 1998 |
|
Chan WS, Ray JG: Low molecular weight heparin use during pregnancy: Issues of safety and practicality. Obstet Gynecol Surv 54:649, 1999 |
|
Tengborn L, Bergqvist D, Matzsch T et al: Recurrent thromboembolism in pregnancy and puerperium: Is there a need for thromboprophylaxis. Am J Obstet Gynecol 160:90, 1989 |
|
Lao TT, deSwiet M, Letsky E et al: Prophylaxis of thromboembolism in pregnancy: An alternative. Br J Obstet Gynaecol 92:202, 1985 |
|
Meschengieser SS, Fondevila CG, Santarelli MT et al: Anticoagulation in pregnant women with mechanical heart valve prostheses. Heart 82:23, 1999 |
|
Iturbe-Alessio I, del Carmen Fonseca M, Mutchinik O et al: Risks of anticoagulant therapy in pregnant women with artificial heart valves. N Engl J Med 315:1390, 1986 |
|
Mujtaba Q, Burrow GN: Treatment of hyperthyroidism in pregnancy with propylthiouracil and methimazole. Obstet Gynecol 46:282, 1975 |
|
Wing DA, Millar LK, Koonings PP et al: A comparison of propylthiouracil versus methimazole in the treatment of hyperthyroidism in pregnancy. Am J Obstet Gynecol 170:90, 1994 |
|
Burrow GN: Thyroid diseases. In: Burrow GN, Ferris TF (eds): Medical Complications During Pregnancy. Philadelphia, WB Saunders, p 229, 1988 |
|
Mandel SJ, Larsen PR, Seely EW et al: Increased need for thyroxine during pregnancy in women with primary hypothyroidism. N Engl J Med 323:91, 1990 |
|
l'Allemand D, Gruters A, Heidemann P et al: Iodine-induced alterations of thyroid function in newborn infants after prenatal and perinatal exposure to povidone iodine. J Pediatr 102:935, 1983 |
|
Czeizel A: Lack of evidence of teratogenicity of benzodiazepine drugs in Hungary. Reprod Toxicol 3:183, 1988 |
|
Laegreid L, Olegard R, Wahlstrom J et al: Abnormalities in children exposed to benzodiazepines in utero. Lancet 1:108, 1987 |
|
Bergman V, Rosa F, Baum C et al: Effects of exposure to benzodiazepine during fetal life. Lancet 340:694, 1992 |
|
Linden S, Rich CL: The use of lithium during pregnancy and lactation. J Clin Psychiatry 44:358, 1983 |
|
Weinstein MR, Goldfield MD: Cardiovascular malformations with lithium use during pregnancy. Am J Psychiatry 132:529, 1975 |
|
Zalzstein E, Koren G, Einarson T et al: A case-control study on the association between first trimest exposure to lithium and Ebstein's anomaly. Am J Cardiol 65:817, 1990 |
|
Jacobson SJ, Jones K, Johnson K et al: Prospective multi-centre study of pregnancy outcome after lithium exposure during first trimester. Lancet 339:530, 1992 |
|
Krause S, Ebbesen F, Lange AP: Polyhydramnios with maternal lithium treatment. Obstet Gynecol 75:504, 1990 |
|
Ang MS, Thorp JA, Parisi VM: Maternal lithium therapy and polyhydramnios. Obstet Gynecol 76:517, 1990 |
|
Cohen LS, Friedman MJ, Jefferson JW: A reevaluation of risk of in utero exposure to lithium. JAMA 271:146, 1994 |
|
Cohen LS, Altshuler LL, Harlow BL et al: Relapse of major depression during pregnancy in women who maintain or discontinue antidepressant treatment. JAMA 295:499, 2006 |
|
Briggs GC, Freeman RK, Jaffe SJ: Drugs in Pregnancy and Lactation. 6th ed. Baltimore, Williams & Wilkins, p 693, 2002 |
|
Briggs GC, Freeman RK, Yaffe SJ: Drugs in Pregnancy and Lactation. 6th ed. Baltimore, Williams & Wilkins, p 60, 2002 |
|
Pastuszak A, Schick-Boschetto B, Zuber C et al: Pregnancy outcome following first-trimester exposure to fluoxetine (Prozac). JAMA 269:2446, 1993 |
|
Goldstein DJ, Corbin LA, Sundell KL: Effects of first-trimester fluoxetine exposure on the newborn. Obstet Gynecol 89:713, 1997 |
|
Nulman I, Rovert J, Stewart DE et al: Neurodevelopment of children exposed in utero to antidepressant drugs. N Engl J Med 336:258, 1997 |
|
Chambers CD, Johnson KA, Dick LM et al: Birth outcomes in pregnant women taking fluoxetine. N Engl J Med 335:1010, 1996 |
|
Kulin NA, Pastuszak A, Sage SR et al: Pregnancy outcome following maternal use of the new selective serotonin reuptake inhibitors: A prospective controlled multicenter study. JAMA 279:609, 1998 |
|
Nulman I, Rovet J, Stewart DE et al: Child development following exposure to tricyclic antidepressants or fluoxetine throughout fetal life: a prospective controlled study. Am J Psych 159:1889, 2002 |
|
Hendrick V, Smith LM, Suri R et al: Birth outcomes after prenatal exposure to antidepressant medication. Am J Obstet Gynecol 188:812, 2003 |
|
Louik C, Lin AE, Werler MM et al: First-trimester use of selective serotonin-reuptake inhibitors and the risk of birth defects. N Engl J Med 356:2675, 2007 |
|
Alwan S, Reefhuis J, Rasmussen SA, et al: Use of selective serotonin-reuptake inhibitors in pregnancy and the risk of birth defects. N Engl J Med 356:2684, 2007 |
|
Chambers CD, Hernandez-Diaz S, Van Marter LJ, et al: Selective serotonin-reuptake inhibitors and risk of persistent pulmonary hypertension of the newborn. N Engl J Med 354:579, 2006 |
|
ACOG Committee on Practice Bulletins No. 92, American College of Obstetricians and Gynecologists: Use of psychiatric medications during pregnancy and lactation. Obstet Gynecol 111:1001, 2008 |
|
Sahakian V, Rouse D, Sipes S et al: Vitamin B6 is effective therapy for nausea and vomiting of pregnancy: A randomized double-blind, placebo-controlled study. Obstet Gynecol 78:33, 1991 |
|
Vutyavanich T, Wongtra-Rjan S, Ruangsri R: Pyridoxine for nausea and vomiting of pregnancy: A randomized double-blind placebo-controlled trial. Am J Obstet Gynecol 173:881, 1995 |
|
Diggory PLC, Tomkinson JS: Nausea and vomiting in pregnancy: A trial of meclozine dihydrochloride with and without pyridoxine. Lancet 2:370, 1962 |
|
Heinonen OP, Slone S, Shapiro S: Birth Defects and Drugs in Pregnancy. Littleton, MA, Publishing Sciences Group, 1977 |
|
Milkovich L, Van Den Berg BJ: An evaluation of the teratogenicity of certain antinauseant drugs. Am J Obstet Gynecol 125:244, 1976 |
|
Cartwright EW: Dramamine in nausea and vomiting of pregnancy. West J Surg Obstet Gynecol 59:216, 1951 |
|
Sorensen HT, Nielsen GL, Christensen K et al: Birth outcomes following maternal use of metoclopramide. Br J Clin Pharmacol 49:264, 2000 |
|
Sullivan CA, Johnson CA, Roach H et al: A pilot study of intravenous ondansetron for hyperemesis gravidarum. Am J Obstet Gynecol 174:2565, 1996 |
|
Safari HR, Fassett MJ, Souter IC et al: The efficacy of methylprednisolone in the treatment of hyperemesis gravidarum: A randomized, double-blind, controlled study. Am J Obstet Gynecol 179:921, 1998 |
|
Park-Wyllie L, Mazzotta P, Pastuszak A et al: Birth defects after maternal exposure to corticosteroids: Prospective cohort study and meta-analysis of epidemiological studies. Teratology 62:385, 2000 |
|
Carmichael SL, Shaw GM: Maternal corticosteroid use and risk of selected congenital anomalies. Am J Med Genet 86:242, 1999 |
|
Vutyavanich T, Kraisarin T, Ruangsri R: Ginger for nausea and vomiting in pregnancy: Randomized, double-masked, placebo-controlled trial. Obstet Gynecol 97:577, 2001 |
|
Fischer-Rasmussen W, Kjaer SK, Dahl C et al: Ginger treatment of hyperemesis gravidarum. Europ J Obstet Gynecol Reprod Biol 138:19, 1990 |
|
Ruigomez A, Garcia Rodriguez LA, Cattaruzzi C et al: Use of cimetidine, omeprazole, and ranitidine in pregnant women and pregnancy outcomes. Am J Epidemiol 150:476, 1999 |
|
Briggs GG, Freeman RK, Yaffe SJ: Drugs in Pregnancy and Lactation. 6th ed.. Baltimore, Williams & Wilkins, 1321, 2002 |
|
Pastuszak A, Schick B, D'Alimonte D et al: The safety of astemizole in pregnancy. J Allergy Clin Immunol 98:748, 1996 |
|
Zierler S, Purohit D: Prenatal antihistamine exposure and retrolental fibroplasia. Am J Epidemiol 123:192, 1986 |
|
Werler MM, Mitchell AA, Shapiro S: First trimester maternal medication use in relation to gastroschisis. Teratology 45:361, 1992 |
|
Werler MM, Mitchell AA, Shapiro S: The relation of aspirin use during the first trimester of pregnancy to congenital cardiac defects. N Engl J Med 321:1639, 1989 |
|
Kozer E, Nikfar S, Costei A et al: Aspirin consumption during the first trimester of pregnancy and congenital anomalies: A meta-analysis. Am J Obstet Gynecol 187:1623, 2002 |
|
Collins E, Turner G: Salicylates and pregnancy. Lancet 2:1494, 1973 |
|
Stuart JJ, Gross SJ, Elrad H et al: Effects of acetylsalicylic acid ingestion on maternal and neonatal hemostasis. N Engl J Med 307:909, 1982 |
|
Areilla RA, Thilenius OB, Ranniger K: Congestive heart failure from suspected ductal closure in utero. J Pediatr 75:74, 1969 |
|
Cowchock FS, Reece EA, Balaban D et al: Repeated fetal losses associated with antiphospholipid antibodies: A collaborative randomized trial comparing prednisone with low-dose heparin treatment. Am J Obstet Gynecol 166:1318, 1992 |
|
Farquharson RG, Quenby S, Greaves M: Antiphospholipid syndrome in pregnancy: A randomized, controlled trial of treatment. Obstet Gynecol 100:408, 2002 |
|
Thulstrup AM, Sorensen HT, Nielsen GL et al: Fetal growth and adverse birth outcomes in women receiving prescriptions for acetaminophen during pregnancy. Am J Perinatol 16:321, 1999 |
|
Waltman T, Tricomi V, Tavakoli FM: Effect of aspirin on bleeding time during elective abortion. Obstet Gynecol 48:108, 1976 |
|
Rayburn W, Shukla U, Stetson P et al: Acetaminophen pharmacokinetics: Comparison between pregnant and nonpregnant women. Obstet Gynecol 155:1353, 1986 |
|
Briggs GG, Freeman RK, Yaffe SJ: Drugs in Pregnancy and Lactation. 6th ed.. Baltimore, Williams & Wilkins, 685–687, 2002 |
|
Briggs GG, Freeman RK, Yaffe SJ: Drugs in Pregnancy and Lactation. 6th ed.. Baltimore, Williams & Wilkins, 171–172, 2002 |
|
Tyson HK: Neonatal withdrawal symptoms associated with maternal use of propoxyphene hydrochloride (Darvon). J Pediatr 85:684, 1974 |
|
Briggs GG, Freeman RK, Yaffe SJ: Drugs in Pregnancy and Lactation. 6th ed.. Baltimore, Williams & Wilkins, 1301, 2002 |
|
Czeizel AE, Rockenbauer M, Sorensen HT et al: Use of cephalosporins during pregnancy and in the presence of congenital abnormalities: A population-based, case-control study. Am J Obstet Gynecol 184:1289, 2001 |
|
Briggs GG, Freeman RK, Yaffe SJ: Drugs in Pregnancy and Lactation. 6th ed.. Baltimore, Williams & Wilkins, 1291–1295, 2002 |
|
Jarnerot G, Into-Malmberg MB, Esbjorner E: Placental transfer of sulphasalazine and sulphapyridine and some of its metabolites. Scand J Gastroenterol 16:693, 1981 |
|
Ochoa AG: Trimethoprim and sulfamethoxazole in pregnancy. JAMA 217:1244, 1971 |
|
Brumfitt W, Pursell R: Double-blind trial to compare ampicillin, cephalexin, co-trimoxazole, and trimethoprim in treatment of urinary infection. Br Med J 2:673, 1972 |
|
Briggs GG, Freeman RK, Yaffe SJ: Drugs in Pregnancy and Lactation. 6th ed. Baltimore, Williams & Wilkins, p 1394, 2002 |
|
Czeizel A: A case-control analysis of the teratogenic effects of co-trimoxazole. Reprod Toxicol 4:305, 1990 |
|
Hernandez-Diaz S, Werler MM, Walker AM et al: Folic acid antagonists during pregnancy and the risk of birth defects. N Engl J Med 343:1608, 2002 |
|
Hailey FJ, Fort H, Williams JR et al: Foetal safety of nitrofurantoin macrocrystals therapy during pregnancy: A retrospective analysis. J Int Med Res 11:364, 1983 |
|
Lenke RR, VanDorsten JP, Schifrin BS: Pyelonephritis in pregnancy: A prospective randomized trial to prevent recurrent disease evaluating suppressive therapy with nitrofurantoin and close surveillance. Am J Obstet Gynecol 146:953, 1983 |
|
Cohlan SQU, Bevelander G, Tiamsic T: Growth inhibition of prematures receiving tetracycline. Am J Dis Child 105:453, 1963 |
|
Aselton P, Jick H, Mulnsky A et al: First-trimester drug use and congenital disorders. Obstet Gynecol 65:451, 1985 |
|
Czeizel AE, Rockenbauer M: Teratogenic study of doxycyline. Obstet Gynecol 89:524, 1997 |
|
Robinson GC, Cambon KG: Hearing loss in infants of tuberculous mothers treated with streptomycin during pregnancy. N Engl J Med 271:949, 1964 |
|
Nishimura H, Tanimura T: Clinical Aspects of the Teratogenicity of Drugs. Amsterdam, Excerpta Medica, 1976 |
|
L'Hommedieu CS, Nicholas D, Armes DA et al: Potentiation of magnesium ulfate-induced neuromuscular weakness by gentamicin, tobramycin, and amikacin. J Pediatr 102:629, 1983 |
|
Marynowski A, Sianozecka E: Comparison of the incidence of congenital malformations in neonates from healthy mothers and from patients treated because of tuberculosis. Ginekol Pol 43:713, 1972 |
|
Zaske DE, Cipolle RJ, Strate RG et al: Rapid gentamicin elimination in obstetric patients. Obstet Gynecol 56:559, 1980 |
|
Mitra AG, Whitten MK, Laurent SL et al: A randomized, prospective study comparing once-daily gentamicin versus thrice-daily gentamicin in the treatment of puerperal infection. Am J Obstet Gynecol 177:786, 1997 |
|
American Thoracic Society: Treatment of tuberculosis and tuberculosis infection in adults and children. Am J Respir Crit Care Med 149:1359, 1994 |
|
Philipson A, Sabath LD, Charles D: Transplacental passage of erythromycin and clindamycin. N Engl J Med 288:1219, 1973 |
|
South MA, Short DH, Knox JM: Failure of erythromycin estolate therapy in in utero syphilis. JAMA 190:70, 1964 |
|
Einarson A, Phillips E, Mawji F et al: A prospective controlled multicentre study of clarithromycin in pregnancy. Am J Perinatol 15:523, 1998 |
|
Briggs GC, Freeman RK, Jaffe SJ: Drugs in Pregnancy and Lactation. 6th ed.. Baltimore, Williams & Wilkins, 286, 2002 |
|
Berkovitch M, Pastuszak A, Gazarian M et al: Safety of the new quinolones in pregnancy. Obstet Gynecol 84:535, 1994 |
|
Briggs GG, Freeman RK, Yaffe SJ: Drugs in Pregnancy and lactation. 6th ed. Baltimore, Williams & Wilkins, p 270, 2002 |
|
Piper JM, Mitchel EF, Ray WA: Prenatal use of metronidazole and birth defects: No association. Obstet Gynecol 82:348, 1993 |
|
Burtin P, Taddio A, Ariburnu O et al: Safety of metronidazole in pregnancy: A meta-analysis. Am J Obstet Gynecol 172:525, 1995 |
|
Culnane M, Fowler MG, Lee SS et al: Lack of long-term effects of in utero exposure to zidovudine among uninfected children born to HIV-infected women. JAMA 281:151, 1999 |
|
McGowan JP, Crane M, Wiznia AA et al: Combination antiretroviral therapy in human immunodeficiency virus-infected pregnant women. Obstet Gynecol 94:641, 1999 |
|
Watts DH: Management of human immunodeficiency virus infection in pregnancy. N Engl J Med 346:1879, 2002 |
|
Rosa FW, Baum C, Shaw M: Pregnancy outcomes after first trimester vaginitis drug therapy. Obstet Gynecol 69:751, 1987 |
|
Briggs GG, Freeman RK, Yaffe SJ: Drugs in Pregnancy and lactation. 6th ed. Baltimore, Williams & Wilkins, p 300, 2002 |
|
Pursley TJ, Blomquist IK, Abraham J et al: Fluconazole-induced congenital anomalies in three infants. Clin Infect Dis 22:336, 1996 |
|
Jick SS: Pregnancy outcomes after maternal exposure to fluconazole. Pharmacotherapy 19:221, 1999 |
|
Mastroiacovo P, Mazzone T, Botto LD et al: Prospective assessment of pregnancy outcomes after first-trimester exposure to fluconazole. Am J Obstet Gynecol 175:1645, 1996 |
|
Main EK, Main DM, Gabbe SG: Chronic oral terbutaline therapy is associated with maternal glucose intolerance. Obstet Gynecol 157:644, 1987 |
|
Weiner CP, Landas S, Persoon TJ: Digoxin-like immunoreactive substance in fetuses with and without cardiac pathology. Am J Obstet Gynecol 157:368, 1987 |
|
Weiner CP, Thompson MIB: Direct treatment of fetal supraventricular tachycardia after failed transplacental therapy. Am J Obstet Gynecol 158:570, 1988 |
|
Pruyn SC, Phelan JP, Buchanan GC: Long-term propranolol therapy in pregnancy: maternal and fetal outcome. Am J Obstet Gynecol 135:485, 1979 |
|
Redmond GP: Propranolol and fetal growth retardation. Semin Perinatol 6:142, 1982 |
|
Magee LA, Bull SB, Kren G et al: The generalizability of trial data; a comparison of β-blocker trial participants with a prospective cohort of women taking β-blockers in pregnancy. Europ J Obstet Gynecol Reprod Biol 94:205, 2001 |
|
Lydakis C, Lip GYH, Beevers M et al: Atenolol and fetal growth in pregnancies complicated by hypertension. Am J Hypertension 12:541, 1999 |
|
Easterling TR, Carr DB, Brateng D et al: Treatment of hypertension in pregnancy: Effect of atenolol on maternal disease, preterm delivery, and fetal growth. Obstet Gynecol 98:427, 2001 |
|
Burrows RF, Burrows EA: Assessing the teratogenic potential of angiotensin-converting enzyme inhibitors in pregnancy. Aust NZJ Obstet Gynecol 38:3:306, 1998 |
|
Hanssens M, Keirse MJNC, Vankelecom F et al: Fetal and neonatal effects of treatment with angiotensin-converting enzyme inhibitors in pregnancy. Obstet Gynecol 78:128, 1991 |
|
Piper JM, Ray WA, Rosa FW: Pregnancy outcome following exposure to angiotensin-converting enzyme inhibitors. Obstet Gynecol 80:429, 1992 |
|
Asch RH, Greenblatt RB: Update on the safety and efficacy of clomiphene citrate as a therapeutic agent. J Reprod Med 17:175, 1976 |
|
Riuz-Velasco V, Tolis G: Pregnancy in hyperprolactinemic women. Fertil Steril 41:793, 1984 |
|
Wiseman RA, Dodds-Smith IC: Cardiovascular birth defects and antenatal exposure to female sex hormones: A reevaluation of some base data. Teratology 30:359, 1984 |
|
Katz Z, Lancet M, Skornik J et al: Teratogenicity of progestagens given during the first trimester of pregnancy. Obstet Gynecol 65:775, 1985 |
|
Raman-Wilms L, Tseng AL, Wighardt S et al: Fetal genital effects of first-trimester sex hormone exposure: A meta-analysis. Obstet Gynecol 85:141, 1995 |
|
Wilkins L: Masculinization of female fetus due to use of orally given progestins. JAMA 172:1028, 1960 |
|
Duck SC, Katayama KP: Danazol may cause female pseudohermaphroditism. Fertil Steril 35:230, 1981 |
|
Shapiro S, Slone D, Heinonen OP et al: Birth defects and vaginal spermicides. JAMA 247:2381, 1982 |
|
Mills JL, Reed GF, Nugent RP et al: Are there adverse effects of periconceptional spermicide use. Fertil Steril 43:442, 1985 |
|
Linn S, Schoenbaum SC, Monson RR et al: Lack of association between contraceptive usage and congenital malformations in offspring. Am J Obstet Gynecol 147:923, 1983 |
|
Shah NR, Bracken MB: A systematic review and meta-analysis of prospective studies on the association between interval cigarette smoking and preterm delivery. Am J Obstet Gynecol 182:465, 2000 |
|
Alberman E, Creasy M, Elliott M et al: Maternal factors associated with fetal chromosomal anomalies in spontaneous abortions. J Obstet Gynecol 83:621, 1976 |
|
Dolan-Mullen P, Ramierez G, Groff JY: A meta-analysis of randomized trials of prenatal smoking cessation interventions. Am J Obstet Gynecol 171:1328, 1994 |
|
Martin TR, Bracken MB: Association of low birth weight with passive smoke exposure in pregnancy. Am J Epidemiol 124:633, 1986 |
|
Marcus BH, Albrecht AE, King TK et al: The efficacy of exercise as an aid for smoking cessation in women. Arch Intern Med 159:1229, 1999 |
|
Tonnesen P, Norregaard J, Simonsen K et al: A double-blind trial of a 16-hour transdermal nicotine patch in smoking cessation. N Engl J Med 325:311, 1991 |
|
Ogburn PL, Hurt RD, Croghan IT et al: Nicotine patch use in pregnant smokers: Nicotine and cotinine levels and fetal effects. Am J Obstet Gynecol 181:736, 1999 |
|
Jones KL, Smith DW, Ulleland CN et al: Patterns of malformation in offspring of chronic alcoholic mothers. Lancet 1:1267, 1973 |
|
Rosett HL: A clinical perspective of the fetal alcohol syndrome. Alcohol Clin Exp Res 4:119, 1980 |
|
Jones KL, Smith DW, Streissguth AP et al: Outcome of offspring of chronic alcoholic women. Lancet 1:1076, 1974 |
|
Ouellette EM, Rosett HL, Rosman NP et al: Adverse effects on offspring of maternal alcohol abuse during pregnancy. N Engl J Med 297:528, 1977 |
|
Mills JL, Graubard BI: Is moderate drinking during pregnancy associated with an increased risk of malformations. Pediatrics 80:309, 1987 |
|
Sokol RJ, Martier SS, Ager JW: The T-ACE questions: Practical prenatal detection of risk-drinking. Am J Obstet Gynecol 160:863, 1989 |
|
Van den Berg BJ: Epidemiologic observations of prematurity: Effects of tobacco, coffee and alcohol. In: Reed DM, Stanley FJ (eds): The Epidemiology of Prematurity. Baltimore, Urban & Schwarzenberg, 1977 |
|
Linn S, Schoenbaum SC, Monson RR et al: No association between coffee consumption and adverse outcomes of pregnancy. N Engl J Med 306:141, 1982 |
|
Martin TR, Bracken MB: The association between low birth weight and caffeine consumption during pregnancy. Am J Epidemiol 126:813, 1987 |
|
Beaulac-Baillargeon L, Desrosiers C: Caffeine-cigarette interaction on fetal growth. Am J Obstet Gynecol 157:1236, 1987 |
|
Munoz LM, Lonnerdal B, Keen CL et al: Coffee consumption as a factor in iron deficiency anemia among pregnant women and their infants in Costa Rica. Am J Clin Nutr 48:645, 1988 |
|
Infante-Rivard C, Fernandez A, Gauthier R et al: Fetal loss associated with caffeine intake before and during pregnancy. JAMA 270:2940, 1993 |
|
Mills JL, Holmes LB, Aarons JH et al: Moderate caffeine use and the risk of spontaneous abortion and intrauterine growth retardation. JAMA 269:593, 1993 |
|
Klebanoff MA, Levine RJ, DerSimonian R et al: Maternal serum paraxanthine, a caffeine metabolite, and the risk of spontaneous abortion. N Engl J Med 341:1639, 1999 |
|
Sturtevant FM: Use of aspartame in pregnancy. Int J Fertil 30:85, 1985 |
|
Finnegan LP, Wapner RJ: Narcotic addiction in pregnancy. In: Niebyl JR (ed): Drug Use in Pregnancy. Philadelphia, Lea & Febiger, p 203, 1988 |
|
Greenland S, Staisch KJ, Brown N et al: The effects of marijuana use during pregnancy: Part I. A preliminary epidemiologic study Am J Obstet Gynecol 143:408, 1982 |
|
Fried PA, Buckingham M, Von Kulmiz P: Marijuana use during pregnancy and perinatal risk factors. Am J Obstet Gynecol 146:992, 1983 |
|
Zuckerman B, Frank DA, Hingson R et al: Effects of maternal marijuana and cocaine use on fetal growth. N Engl J Med 320:762, 1989 |
|
Acker D, Sachs BP, Tracey KJ et al: Abruptio placentae associated with cocaine use. Am J Obstet Gynecol 146:220, 1983 |
|
Little BB, Snell LM, Klein VR et al: Cocaine abuse during pregnancy: Maternal and fetal implications. Obstet Gynecol 73:157, 1989 |
|
Bingol N, Fuchs M, Diaz V et al: Teratogenicity of cocaine in humans. J Pediatr 110:93, 1987 |
|
MacGregor SN, Keith LG, Chasnoff IJ et al: Cocaine use during pregnancy: Adverse perinatal outcome. Am J Obstet Gynecol 157:686, 1987 |
|
Keith LG, MacGregor S, Friedell S et al: Substance abuse in pregnant women: Recent experience at the perinatal center for chemical dependence of Northwestern Memorial Hospital. Obstet Gynecol 73:715, 1989 |
|
Chasnoff IJ, Griffith DR, MacGregor S et al: Temporal patterns of cocaine use in pregnancy. JAMA 261:1741, 1989 |
|
Volpe JJ: Effect of cocaine use on the fetus. N Engl J Med 327:399, 1992 |
|
Handler A, Kistin N, Davis F et al: Cocaine use during pregnancy: Perinatal outcomes. Am J Epidemiol 133:818, 1991 |
|
Little BB, Snell LM: Brain growth among fetuses exposed to cocaine in utero Asymmetrical growth retardation. Obstet Gynecol 77:361, 1991 |
|
Chasnoff IJ, Chisum GM, Kaplan WE: Maternal cocaine use and genitourinary tract malformations. Teratology 37:201, 1988 |